
C57BL/6JNifdc-Apptm1(APP*K670N*M671L*V717I )Bcgen /Bcgen • 113380

Gene targeting strategy for B-hAPP*K670N*M671L*V717I mice. The CDS that encodes human APP signal peptide and extracellular domain with Swedish (K670N, M671L) and London (V717I) mutation, followed by human 3’UTR-STOP is inserted right after mouse App 5’UTR to replace the exon 1 of mouse App gene. The APP protein expression will be driven by endogenous mouse App promoter, while mouse App gene transcription and translation will be disrupted.
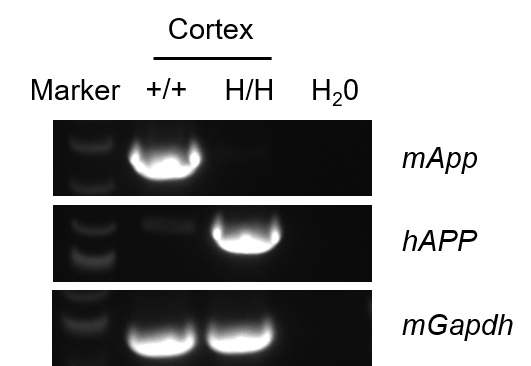
Strain specific analysis of APP mRNA expression in wild-type C57BL/6JNifdc mice and homozygous B-hAPP*K670N*M671L*V717I mice by RT-PCR. Cortex RNA was isolated from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hAPP*K670N*M671L*V717I mice (H/H), then cDNA libraries were synthesized by reverse transcription, followed by PCR with mouse or human APP primers. Mouse App mRNA was detectable in wild-type mice. Human APP mRNA was detectable only in homozygous B-hAPP*K670N*M671L*V717I mice, but not in wild-type mice.

Western blot analysis of APP protein expression in homozygous B-hAPP*K670N*M671L*V717I mice. Various tissue lysates were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hAPP*K670N*M671L*V717I mice (H/H), and then analyzed by western blot with species-specific anti-APP antibody (abcam, ab133588). 50 μg total proteins were loaded for western blotting analysis. Human APP was detected in brain from homozygous B-hAPP*K670N*M671L*V717I mice but not in wild-type mice.
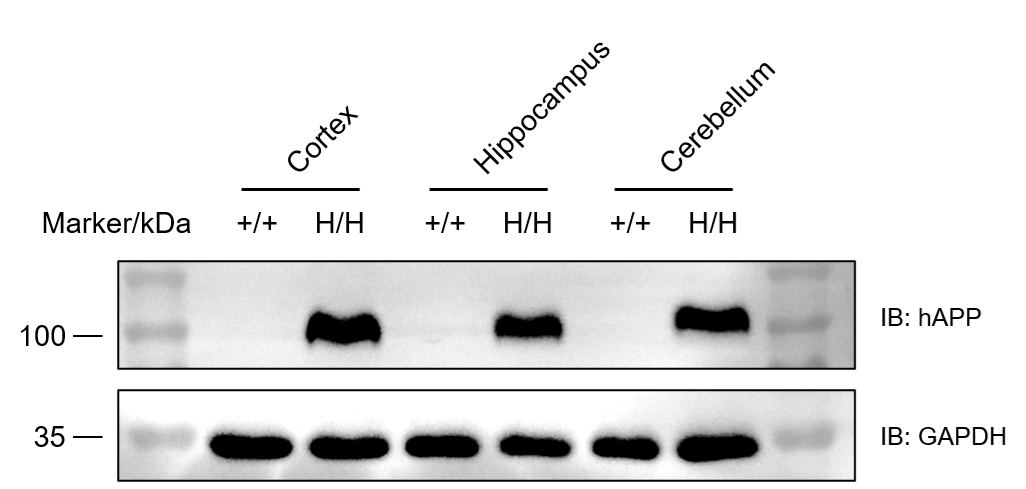
Western blot analysis of APP protein expression in homozygous B-hAPP*K670N*M671L*V717I mice. Various tissue lysates were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hAPP*K670N*M671L*V717I mice (H/H), and then analyzed by western blot with species-specific anti-APP antibody (abcam, ab133588). 50 μg total proteins were loaded for western blotting analysis. Human APP was detected in cortex, hippocampus and cerebellum from homozygous B-hAPP*K670N*M671L*V717I mice but not in wild-type mice.

Western blot analysis of APP protein expression in homozygous B-hAPP*K670N*M671L*V717I mice. Various tissue lysates were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hAPP*K670N*M671L*V717I mice (H/H), and then analyzed by western blot with species-specific anti-APP antibody (Abcam, ab133588). 40 μg total proteins were loaded for western blotting analysis. Human APP was only detected in cortex, hippocampus and spinal cord from homozygous B-hAPP*K670N*M671L*V717I mice but not in wild-type mice. There was no significant difference in the expression of human APP between female and male mice from homozygous B-hAPP*K670N*M671L*V717I mice. M, Male; F, Female.
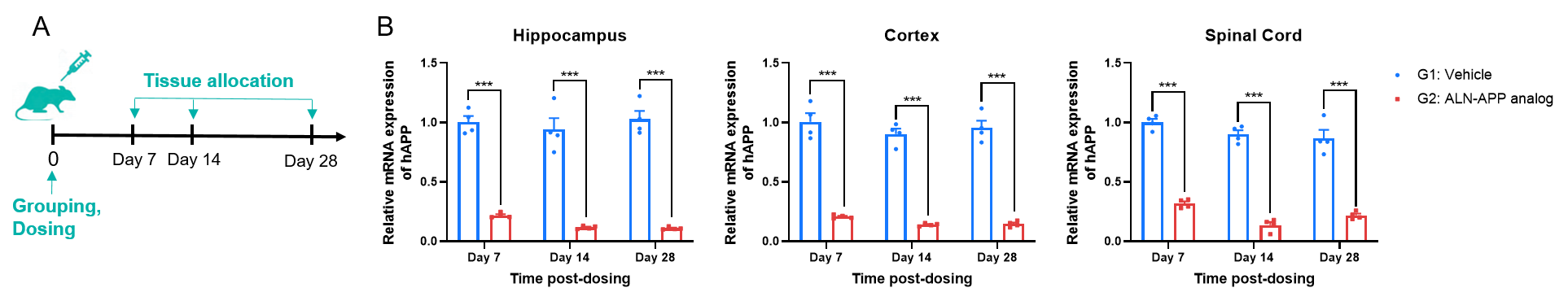
The inhibitory efficiency of the siRNA drugs against human APP in B-hAPP*K670N*M671L*V717I mice. B-hAPP*K670N*M671L*V717I mice were randomly divided into two groups (n=4/group, 10-week-old, male). The ALN-APP analog (provide by client) and vehicle were administered to the mice individually by intra-cerebroventricular injection (ICV). The mice were sacrificed on day 7, day 14 and day 28, respectively. Then the hippocampus, cortex and spinal cord tissue were collected to detect the human APP mRNA by qRT-PCR. (A) The schematic diagram of experimental processing. (B) The expression of human APP mRNA in hippocampus, cortex and spinal cord. The human APP mRNA in the treatment group (G2) was significantly reduced compared to the control group (G1), demonstrating that B-hAPP*K670N*M671L*V717I mice provide a powerful preclinical model for in vivo evaluation of human APP targeted nucleic acid drugs. Values are expressed as mean ± SEM. Significance was determined by unpaired t test. ***P < 0.001.
This experiment was conducted in collaboration with the client using B-hAPP*K670N*M671L*V717I mice.