
C57BL/6JNifdc-Giprtm2(GIPR)Bcgen/Bcgen • 112714
The gastric inhibitory polypeptide receptor (GIPR) belongs to the G protein-coupled receptor family and is expressed in many tissues including the pancreas, stomach, brain, liver, etc. It plays a crucial role in regulating insulin secretion, glucose, and lipid metabolism. Mutations within this gene are associated with health conditions including obesity and diabetes.
In GIPR humanized mice (B-hGIPR), exons 2-14 of the mouse Gipr gene (encoding the entire protein from ATG to STOP codon, including the 3' UTR) were replaced by the human GIPR CDS and 3' UTR. The human GIPR expression is driven by the retained endogenous mouse promoter and 5' UTR, while mouse gene transcription and translation are disrupted.
Validation confirms that human GIPR mRNA is exclusively detectable in GIPR humanized mice (B-hGIPR) across key metabolic tissues such as brain, epididymal white adipose tissue (eWAT), and inguinal white adipose tissue (ingWAT). Functional studies demonstrate that recombinant human GIP effectively promotes insulin secretion during intraperitoneal glucose tolerance tests (IPGTT) in this model. Furthermore, in high-fat diet (HFD)-induced obese GIPR humanized mice (B-hGIPR), anti-human GIPR antibodies (e.g., Amgen 2G10 analog), alone or in combination with Semaglutide, significantly reduce body weight, decrease food intake, and improve insulin resistance, validating the model's utility for metabolic drug evaluation.
Key Advantages
Validation
Applications
In vivo efficacy, pharmacodynamics, and safety evaluation of GIPR-targeting agonists, antagonists, and related combination therapies for metabolic diseases.
In the GIPR humanized mice (B-hGIPR), the genomic region spanning exons 2 to 14 of the mouse Gipr gene—which encodes the full-length protein from the ATG start codon to the STOP codon, including the 3′ UTR—was replaced by the human GIPR coding sequence (CDS) and 3′ UTR. The endogenous mouse promoter and 5′ UTR regions are retained, ensuring that human GIPR expression is driven by the native mouse regulatory elements, while transcription and translation of the mouse Gipr gene are effectively disrupted.
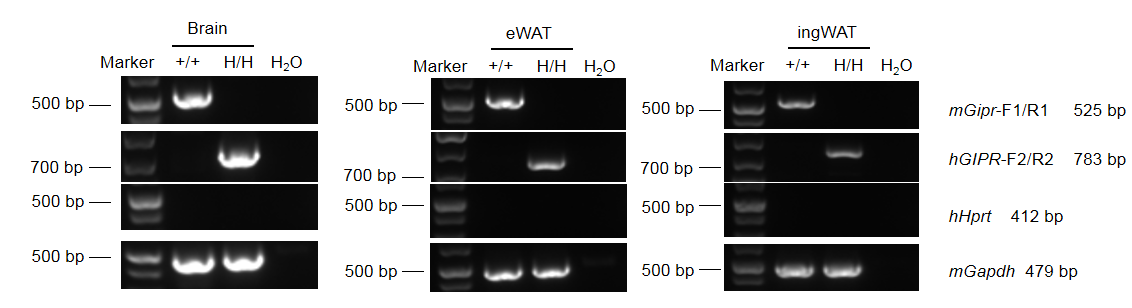
Strain-specific analysis of GIPR gene expression was performed in wild-type C57BL/6 mice and GIPR humanized mice (B-hGIPR) using RT-PCR. RNA was isolated from brain, epididymal white adipose tissue (eWAT), and inguinal white adipose tissue (ingWAT). Mouse Gipr mRNA was detectable in wild-type mice. In contrast, human GIPR mRNA was exclusively detectable in homozygous GIPR humanized mice (H/H) but not in wild-type controls.
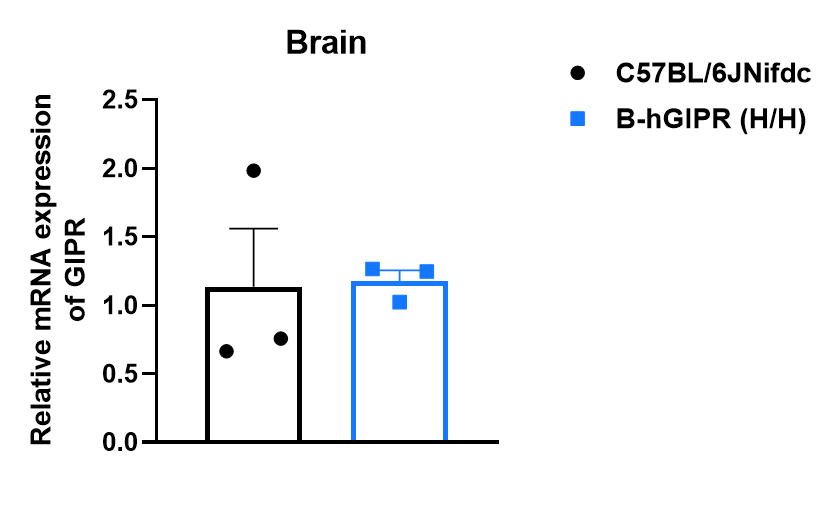
Strain-specific analysis of GIPR mRNA expression was performed in wild-type C57BL/6JNifdc and homozygous GIPR humanized mice (B-hGIPR, H/H) using RT-qPCR. Following reverse transcription, qPCR was conducted with primers targeting the 5' UTR region, enabling detection of both mouse and human GIPR transcripts. mRNA expression levels were comparable between the GIPR humanized mice (B-hGIPR) and wild-type controls (values expressed as mean ± SEM).
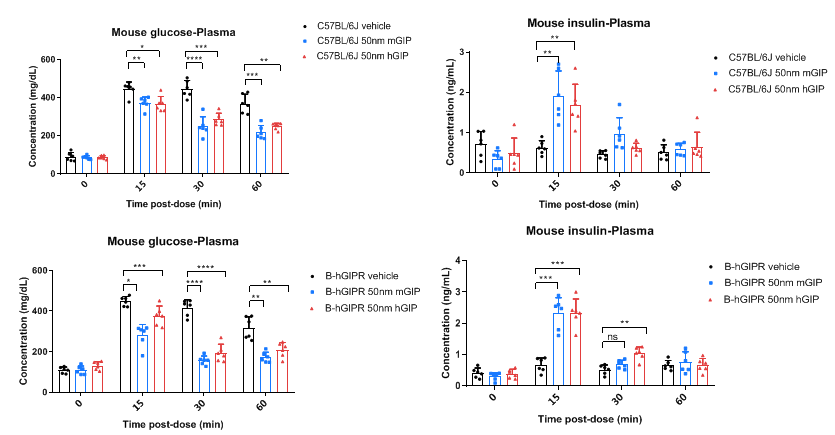
In vivo function of mouse and human GIP was assessed in GIPR humanized mice (B-hGIPR) during an intraperitoneal glucose tolerance test (IPGTT). Plasma glucose and insulin concentrations were measured in wild-type C57BL/6J and homozygous GIPR humanized mice (B-hGIPR) (male, 6-week-old, n=6) following co-administration of glucose (40%) with mouse GIP (HY-P77948) or human GIP (HY-P0276) at 50 nmol/kg (i.p.). In GIPR humanized mice (B-hGIPR), human GIP stimulated insulin secretion and enhanced glucose clearance. Data represent means ± SEM. Analyzed by 2way-ANOVA, *p < 0.05, **p < 0.01,***p < 0.001 ,****p < 0.0001.
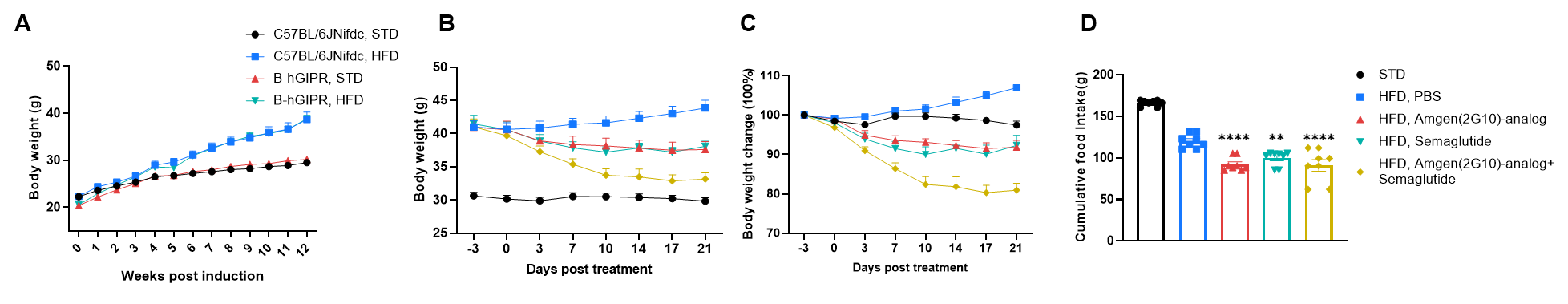
In vivo efficacy of a human GIPR antibody and semaglutide on body weight and food intake was evaluated in high-fat diet (HFD)–induced GIPR humanized mice (B-hGIPR). GIPR humanized mice (B-hGIPR) were fed a high-fat diet for 12 weeks to induce obesity prior to treatment. (A) Body weight changes during HFD induction were monitored to confirm obesity development in GIPR humanized mice (B-hGIPR). (B–C) Following obesity induction, GIPR humanized mice (B-hGIPR) were treated with a human GIPR antibody (Amgen 2G10 analog, in-house), semaglutide, or a combination therapy, and longitudinal body weight changes were assessed. (D) The effects of the human GIPR antibody (Amgen 2G10 analog), semaglutide, and combination treatment on cumulative food intake were evaluated on Day 21. n = 8 mice per group. Data are presented as mean ± SEM. Statistical significance was determined using ordinary one-way ANOVA, with comparisons made against the HFD PBS control group (*p < 0.05, **p < 0.01,***p < 0.001 ,****p < 0.0001).
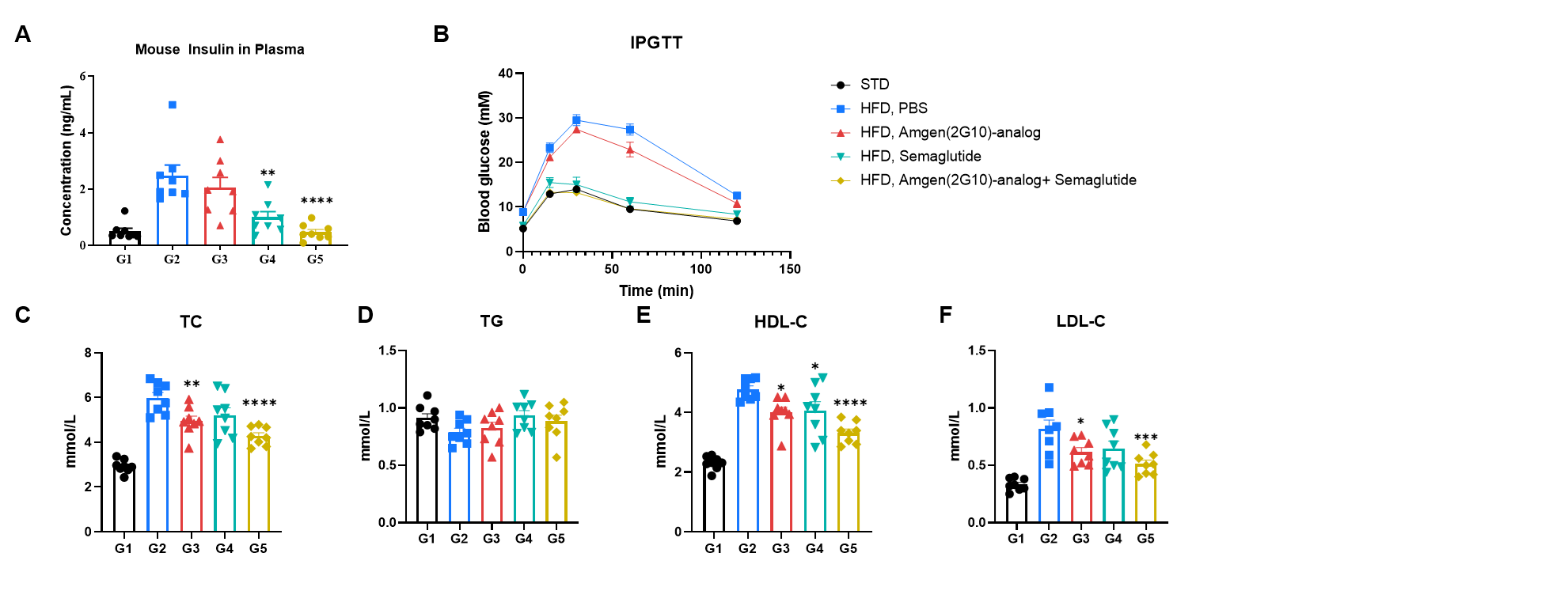
In vivo efficacy of a human GIPR antibody and semaglutide on glucose and blood biochemistry was evaluated in HFD–induced GIPR humanized mice (B-hGIPR). (A) Plasma fasting insulin levels were measured after treatment following 6 hours of fasting. (B) After a 6-hour fast, mice were intraperitoneally injected with 15% glucose (1.5 g/kg) to perform glucose tolerance tests (GTT). (C–F) Blood biochemical parameters were analyzed following treatment. Data are presented as mean ± SEM. Statistical significance was assessed using ordinary one-way ANOVA, with comparisons made against the HFD PBS control group (*p < 0.05, **p < 0.01,***p < 0.001 ,****p < 0.0001).
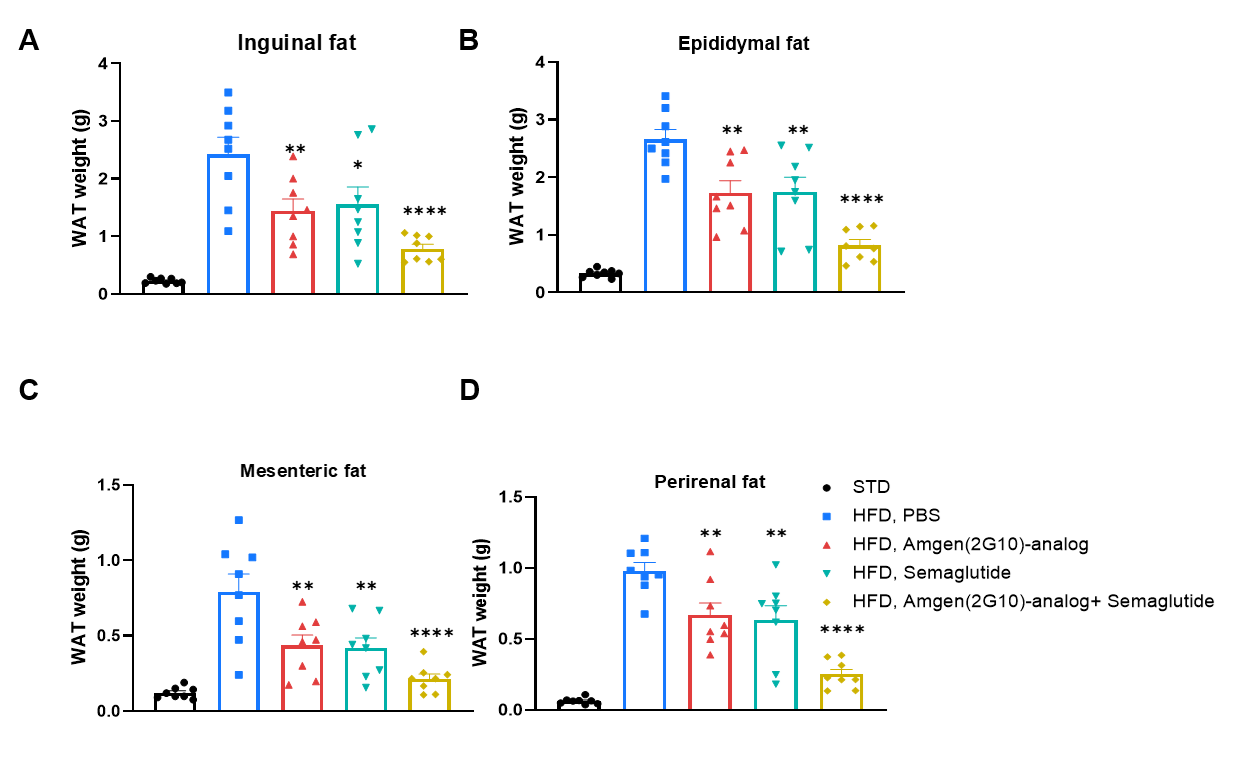
In vivo efficacy of a human GIPR antibody and semaglutide on white adipose tissue reduction in HFD-induced GIPR humanized mice (B-hGIPR). (A–D) The weight of white adipose tissue was measured in HFD-induced GIPR humanized mice (B-hGIPR) at the end of treatment. Values are expressed as mean ± SEM. Significance was determined by Ordinary one-way ANOVA compared with the HFD PBS group (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
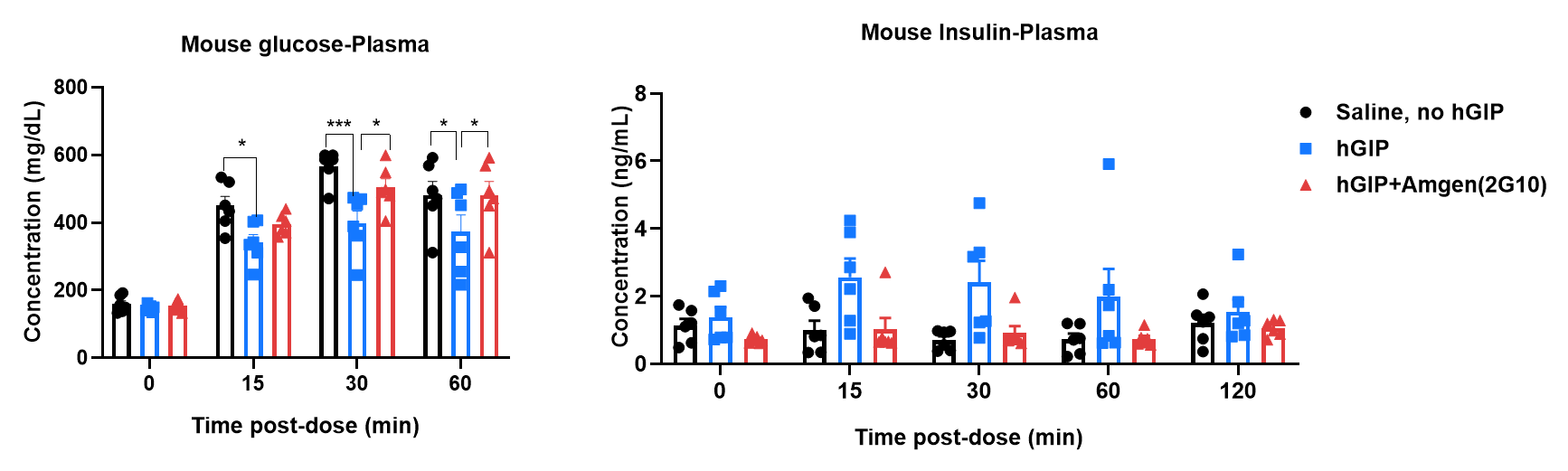
In vivo functional analysis of human GIP and anti-GIPR antibody in HFD-induced GIPR humanized mice (B-hGIPR). GIPR Humanized Mice (B-hGIPR) mice were fed a high-fat diet for 12 weeks to induce obesity, then randomized into treatment groups receiving vehicle or a GIPR antibody (Amgen 2G10 analog, in-house). Approximately 24 hours later, mice were administered saline or 0.25 mg/kg human GIP (hGIP, Cat. HY-P0276, MCE) via intraperitoneal injection, immediately followed by an intraperitoneal injection of glucose (1.5 g/kg). Blood glucose and plasma insulin levels were measured during intraperitoneal glucose tolerance tests (IPGTT). Data are presented as mean ± SEM. Statistical analysis was performed using two-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001).
Q1: What are B-hGIPR mice?
B-hGIPR mice are a humanized model in which the genomic region of the mouse Gipr gene (exons 2-14, encoding the full-length protein including the 3' UTR) has been replaced by the human GIPR coding sequence and 3' UTR. This enables the exclusive expression of the human gastric inhibitory polypeptide receptor (GIPR) under the control of the endogenous mouse promoter.
Q2: Why is the GIPR humanized mouse model important for metabolic research?
GIPR is a key receptor for the incretin hormone GIP, which regulates insulin secretion, glucose homeostasis, and lipid metabolism. This humanized model allows for the preclinical evaluation of therapeutics targeting the human GIPR pathway, which is relevant for conditions like type 2 diabetes and obesity.
Q3: How was the human GIPR gene knocked in?
The targeting strategy involved replacing the region from exon 2 to exon 14 of the mouse Gipr gene (spanning ATG to STOP codon, including the 3' UTR) with the human GIPR CDS and 3' UTR. The mouse promoter and 5' UTR regions are retained to drive physiological expression, while the endogenous mouse gene's transcription and translation are disrupted.
Q4: How is human GIPR expression and function validated in this model?
Human GIPR mRNA is exclusively detectable in homozygous B-hGIPR mice in key metabolic tissues like brain, epididymal white adipose tissue (eWAT), and inguinal white adipose tissue (ingWAT). Functional validation through intraperitoneal glucose tolerance tests (IPGTT) confirms that human GIP effectively stimulates insulin secretion in this model.
Q5: What are the main applications of the B-hGIPR mouse model?
This model is primarily used for the in vivo efficacy, pharmacodynamics, and safety evaluation of GIPR-targeting therapeutics. Applications include studying drug effects on insulin secretion, body weight regulation, and glucose metabolism, particularly in the context of obesity and metabolic syndrome.