
C57BL/6-Il12rb1tm2(IL12RB1)Bcgen Il12rb2tm2(IL12RB2)Bcgen/Bcgen • 112518
Gene targeting strategy for B-hIL12RB1 plus/hIL12RB2 plus mice (112518). IL12RB1: the extracellular region sequences of mouse Il12rb1 were replaced by human IL12RB1 counterpart gene sequences. IL12RB2: the extracellular and transmembrane region sequences of mouse Il12rb2 were replaced by human IL12RB2 counterpart gene sequences.
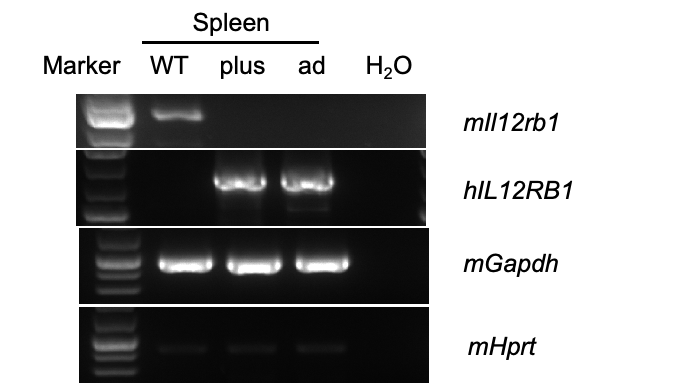
Strain specific analysis of IL12RB1 mRNA expression by RT-PCR. Spleen RNA were isolated from wild-type C57BL/6 mice (WT), homozygous B-hIL12RB1 plus/hIL12RB2 plus mice (plus) and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice (ad), then cDNA libraries were synthesized by reverse transcription, followed by PCR with mouse and human strain specific IL12RB1 primers. Human IL12RB1 mRNA exclusively detectable in homozygous B-hIL12RB1 plus/hIL12RB2 plus mice and B-hIL12RB1 plus/hIL12RB2 ad mice but not in wild-type C57BL/6 mice. Mouse Il12rb1 was only detectable in wild-type C57BL/6 mice but not in humanized mice.
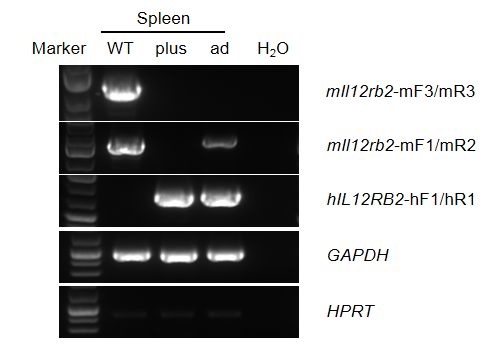
Strain specific analysis of IL12RB2 mRNA expression by RT-PCR.
Spleen RNA were isolated from wild-type C57BL/6 mice (WT), homozygous B-hIL12RB1 plus/hIL12RB2 plus mice (plus) and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice (ad), then cDNA libraries were synthesized by reverse transcription, followed by PCR with mouse and human strain specific IL12RB2 primers.
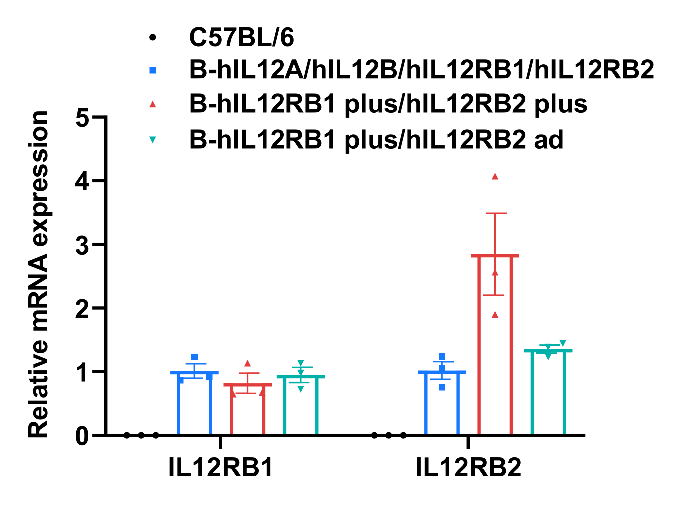
IL12RB1 and IL12RB2 gene expression in wild-type C57BL/6 mice and homozygous B-hIL12RB1/hIL12RB2 mice by RT-qPCR. Splenocytes were collected from wild-type C57BL/6 mice (2 female, 1 male, n=3, 14-week-old), homozygous B-hIL12RB1 plus/hIL12RB2 plus mice (male, n=3, 14-week-old) and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice (male, n=3, 14-week-old). Then cDNA libraries were synthesized by reverse transcription, followed by PCR with human-specific IL12RB1 and IL12RB2 primers. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
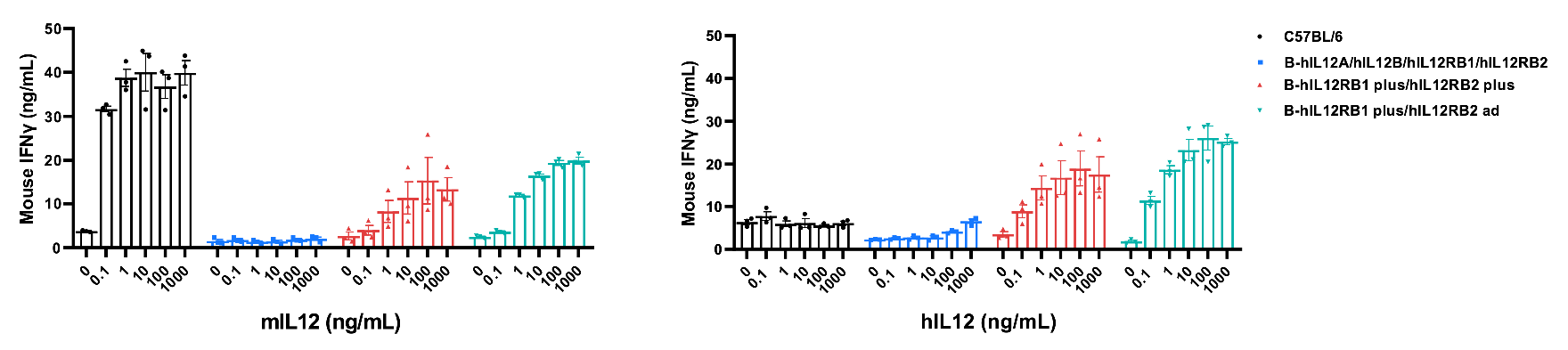
IL12 induced the IFNγ production in CD4+ T cells sorted from splenocytes. CD4+ T cells were sorted from the splenocytes of wild-type C57BL/6 mice, homozygous B-hIL12A/hIL12B/hIL12RB1/hIL12RB2 mice, homozygous B-hIL12RB1 plus/hIL12RB2 plus mice and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice. The production of IFNγ in supernatants were assessed after incubation with mouse IL12 (mIL12) or human IL12 (hIL12) in combination with bead-associated 0.4 μg/mL anti-mCD3e and 0.8 μg/mL anti-mCD28 antibodies for 48 hours. Mouse IFNγ were increased after responsiveness to mIL12 in humanized mice and wild-type mice. While, only hIL12 induced mouse IFNγ increase in humanized mice.
Mice information: 1) humanized mice, male, 3 mice/group, 14-week-old. 2) wild-type C57BL/6 mice, 2 female and 1 male mice, 14-week-old.
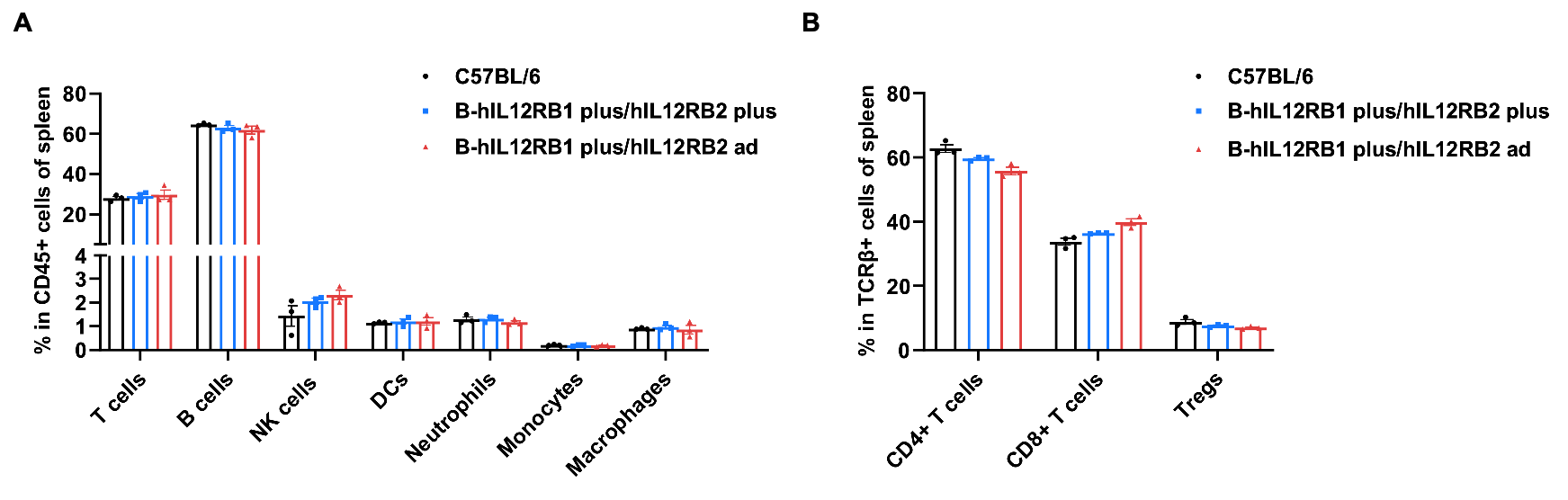
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6 mice (female, n=3, 6-week-old), homozygous B-hIL12RB1 plus/hIL12RB2 plus mice (female, n=3, 9-week-old) and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice (female, n=3, 6-week-old). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes, macrophages, CD4+ T cells, CD8+ T cells and Tregs in B-hIL12RB1 plus/hIL12RB2 plus mice and B-hIL12RB1 plus/hIL12RB2 ad mice were similar to those in C57BL/6 mice. Values are expressed as mean ± SEM. Significance was determined by Multiple t tests. *P < 0.05, **P < 0.01, ***p < 0.001.
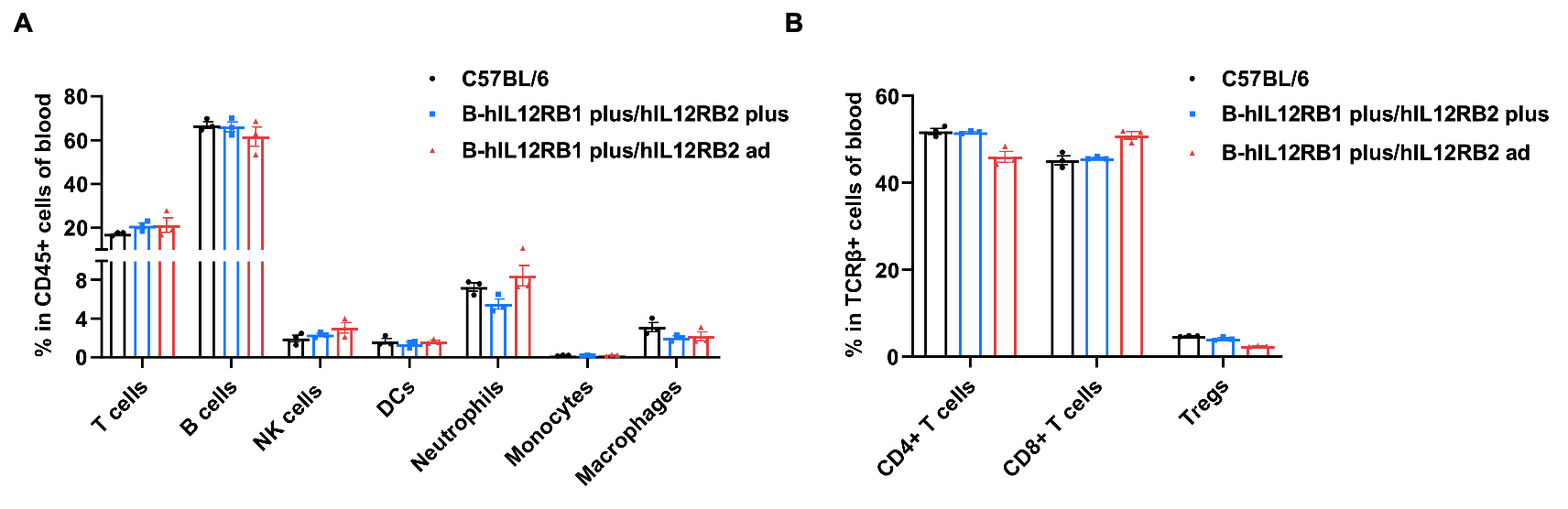
Frequency of leukocyte subpopulations in blood by flow cytometry. Blood cells were isolated from wild-type C57BL/6 mice (female, n=3, 6-week-old), homozygous B-hIL12RB1 plus/hIL12RB2 plus mice (female, n=3, 9-week-old) and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice (female, n=3, 6-week-old). A. Flow cytometry analysis of the blood cells was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes, macrophages, CD4+ T cells, CD8+ T cells and Tregs in B-hIL12RB1 plus/hIL12RB2 plus mice and B-hIL12RB1 plus/hIL12RB2 ad mice were similar to those in C57BL/6 mice. Values are expressed as mean ± SEM. Significance was determined by Multiple t tests. *P < 0.05, **P < 0.01, ***p < 0.001.
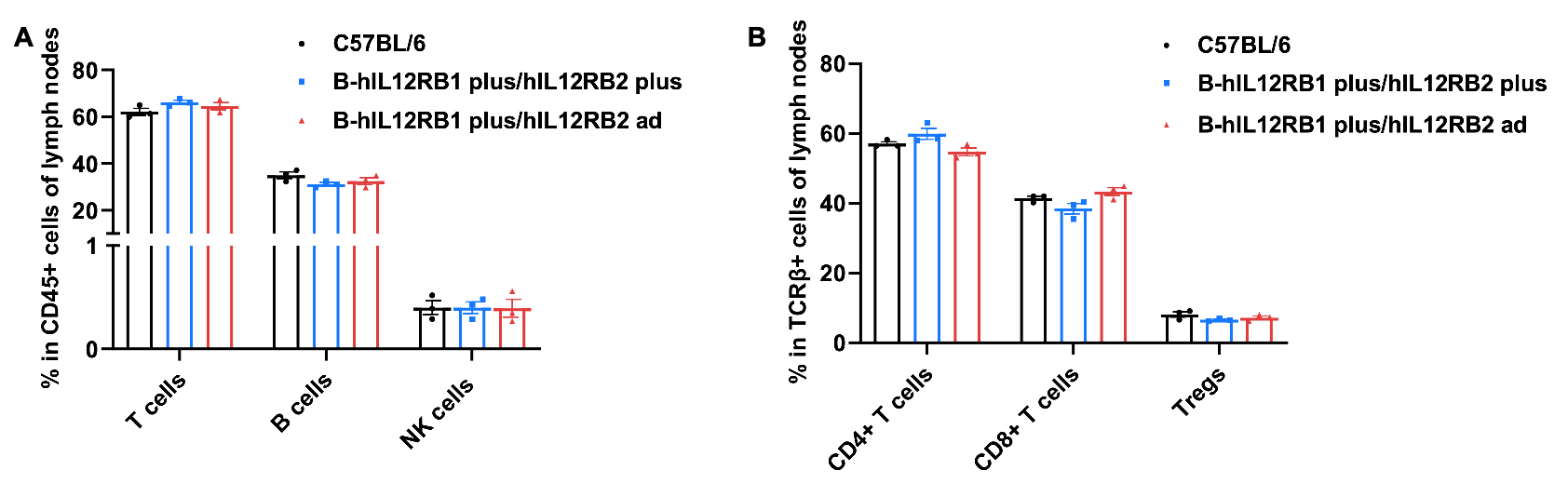
Frequency of leukocyte subpopulations in lymph nodes by flow cytometry. Lymph nodes cells were isolated from wild-type C57BL/6 mice (female, n=3, 6-week-old), homozygous B-hIL12RB1 plus/hIL12RB2 plus mice (female, n=3, 9-week-old) and homozygous B-hIL12RB1 plus/hIL12RB2 ad mice (female, n=3, 6-week-old). A. Flow cytometry analysis of the lymph nodes cells was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, CD4+ T cells, CD8+ T cells and Tregs in B-hIL12RB1 plus/hIL12RB2 plus mice and B-hIL12RB1 plus/hIL12RB2 ad mice were similar to those in C57BL/6 mice. Values are expressed as mean ± SEM. Significance was determined by Multiple t tests. *P < 0.05, **P < 0.01, ***p < 0.001.
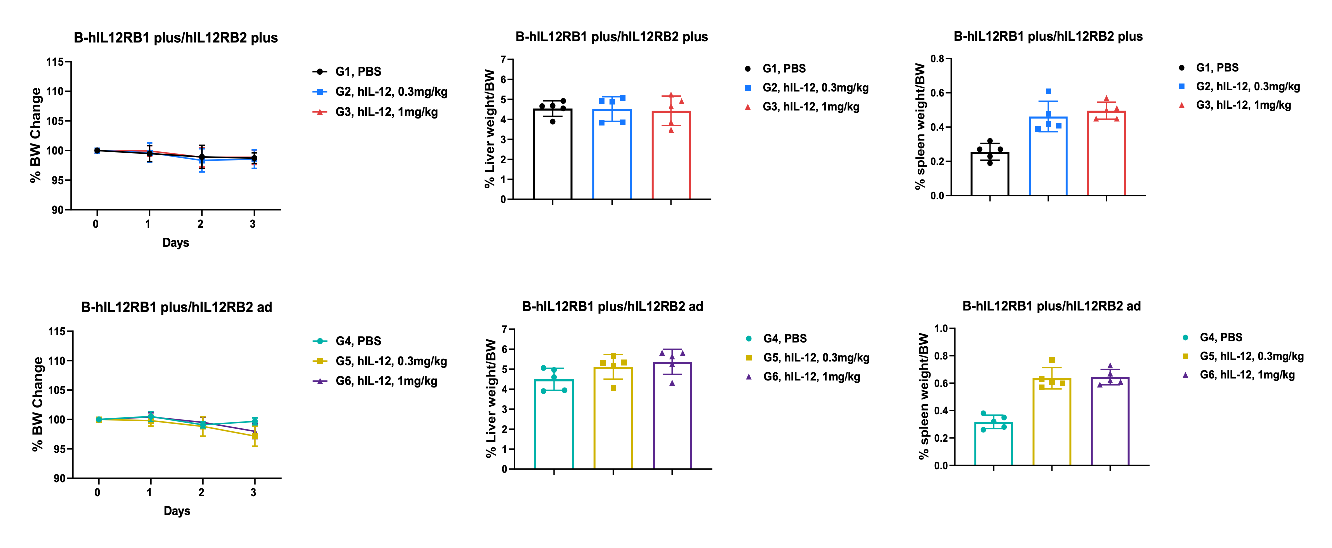
Values are expressed as mean ± SEM. Significance was determined by One-way or Two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
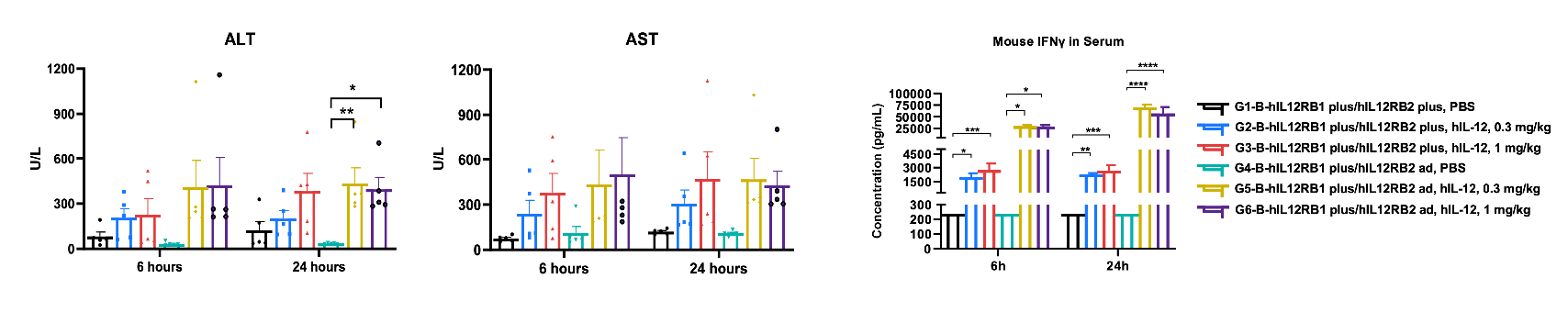
Values are expressed as mean ± SEM. Significance was determined by One-way or Two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.