
C57BL/6-Pcsk9tm1(PCSK9)Bcgen/Bcgen • 110928
Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays a key regulatory role in lipid metabolism by promoting LDL receptor (LDLR) degradation and increasing circulating LDL-cholesterol. PCSK9 has become a major therapeutic target in cardiovascular disease, and PCSK9 humanized mice enable direct evaluation of anti-human PCSK9 therapeutics in vivo.
PCSK9 humanized mice were generated by replacing the endogenous mouse Pcsk9 gene with the human PCSK9 gene. Homozygous mice exclusively express human PCSK9 mRNA and protein, with complete loss of mouse Pcsk9 expression. Lipid metabolism parameters—including TG, TC, HDL-C, and LDL-C—remain comparable to those of wild-type mice, ensuring a physiologically stable background for drug evaluation.
This model provides a robust platform for assessing the mechanism of action and efficacy of anti-human PCSK9 antibodies, LDL cholesterol–lowering potential, and downstream LDLR regulation, making it a highly translational model for cardiovascular drug discovery.
Key Advantages
Validation
Applications
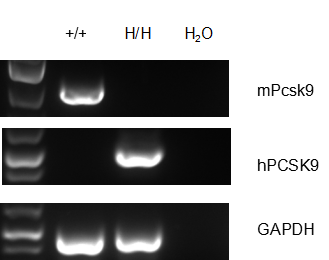
Strain-specific PCSK9 expression was analyzed by RT-PCR in wild-type and PCSK9 humanized mice. Mouse Pcsk9 mRNA was detectable in the livers of wild-type mice (+/+). Human PCSK9 mRNA was detectable only in homozygous B-hPCSK9 mice (H/H) and not in wild-type mice.
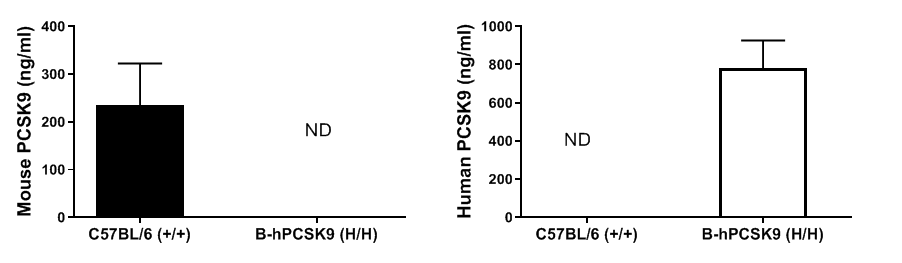
Serum PCSK9 levels were analyzed by ELISA in wild-type (+/+) and homozygous PCSK9 humanized mice (H/H) using species-specific PCSK9 kits. Mouse PCSK9 was detectable in wild-type mice, whereas human PCSK9 was exclusively detectable in PCSK9 humanized mice.
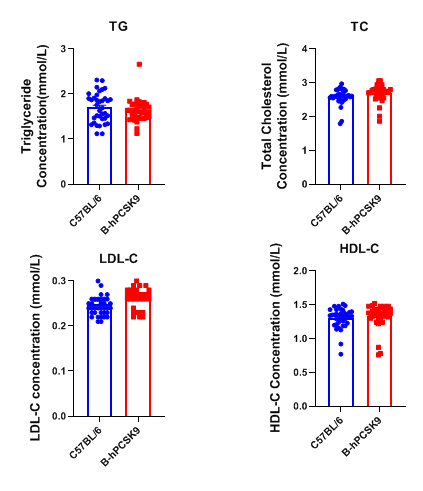
Plasma TG, TC, LDL-C, and HDL-C levels were measured in PCSK9 humanized mice and wild-type C57BL/6 mice (n = 36, 6-week-old). No significant differences were observed between the two strains. TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
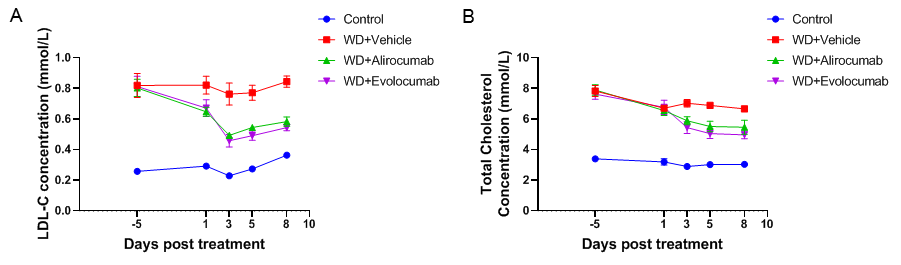
Anti-human PCSK9 antibody improved lipid metabolism in male PCSK9 humanized mice. Wild-type C57BL/6 and PCSK9 humanized male mice were treated with alirocumab (in-house), evolocumab (in-house), or isotype control (single s.c. dose, n=8). Blood was collected on Days -5, 1, 3, 5, and 8. Anti-human PCSK9 antibody treatment significantly reduced LDL-C (A) and TC (B) levels in B-hPCSK9 mice compared with controls. WD: western diet. Values are expressed as mean ± SEM.
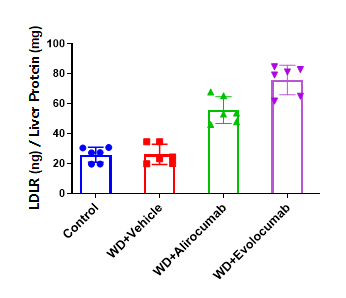
Anti-human PCSK9 antibody upregulated LDLR levels in male PCSK9 humanized mice. B-hPCSK9 male mice were treated with alirocumab (in-house), evolocumab (in-house), or isotype control (single s.c. dose, n=6). Liver tissues collected on Day 8 were analyzed by ELISA. LDLR levels were upregulated in anti-PCSK9–treated mice compared with control. Values are expressed as mean ± SEM. LDLR: low-density lipoprotein receptor; WD: Western diet.
Q1: What makes PCSK9 humanized mice valuable for PCSK9 drug development?
They exclusively express human PCSK9, enabling accurate in vivo evaluation of anti-human PCSK9 therapeutic antibodies.
Q2: Do PCSK9 humanized mice show normal lipid metabolism?
Yes. Triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels remain comparable to those of wild-type mice, providing a stable baseline for lipid-lowering drug studies.
Q3: Can PCSK9 humanized mice be used to evaluate clinical PCSK9 antibodies?
Yes. These mice show robust LDL-C and TC reduction and increased hepatic LDLR expression following treatment with anti-human PCSK9 antibodies such as alirocumab or evolocumab.
Q4: Do PCSK9 humanized mice express mouse Pcsk9?
No. Mouse Pcsk9 is completely absent; only human PCSK9 mRNA and protein are expressed.
Q5: What therapeutic pathways can be studied using this model?
PCSK9–LDLR pathway biology, lipid-lowering mechanisms, antibody pharmacodynamics, and preclinical cardiovascular drug evaluation.