
C57BL/6-Tractm1(TRAC)Bcgen Trbc1tm1(TRBC1)Bcgen Trbc2tm1(TRBC2)Bcgen/Bcgen • 113570
Gene targeting strategy for B-hTRAC/hTRBC1/hTRBC2 mice. The partial exon1, exon 2, partial exon3 and intron 1-2 of mouse Trac were replaced by partial exon1, exon 2, partial exon3 and intron 1-2 of human TRAC in the B-hTRAC/hTRBC1/hTRBC2 mice. The exon1-2, partial exon 3 and intron 1-2 of mouse Trbc1 and Trbc2 were replaced by human exon1-2, partial exon 3 and intron 1-2 of human TRBC1 and TRBC2 in B-hTRAC/hTRBC1/hTRBC2 mice.
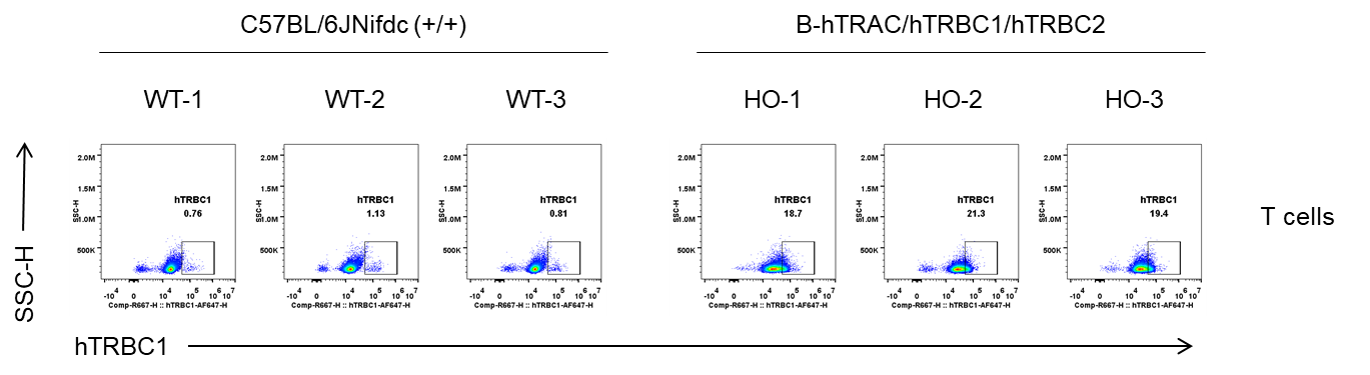
Strain specific TRBC1 expression analysis in homozygous B-hTRAC/hTRBC1/hTRBC2 mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6JNifdc (+/+) and homozygous B-hTRAC/hTRBC1/hTRBC2 mice (H/H), and analyzed by flow cytometry with species-specific anti-human TRBC1 antibody (Biolegend, 383502). Human TRBC1 was exclusively detectable in homozygous B-hTRAC/hTRBC1/hTRBC2 mice.
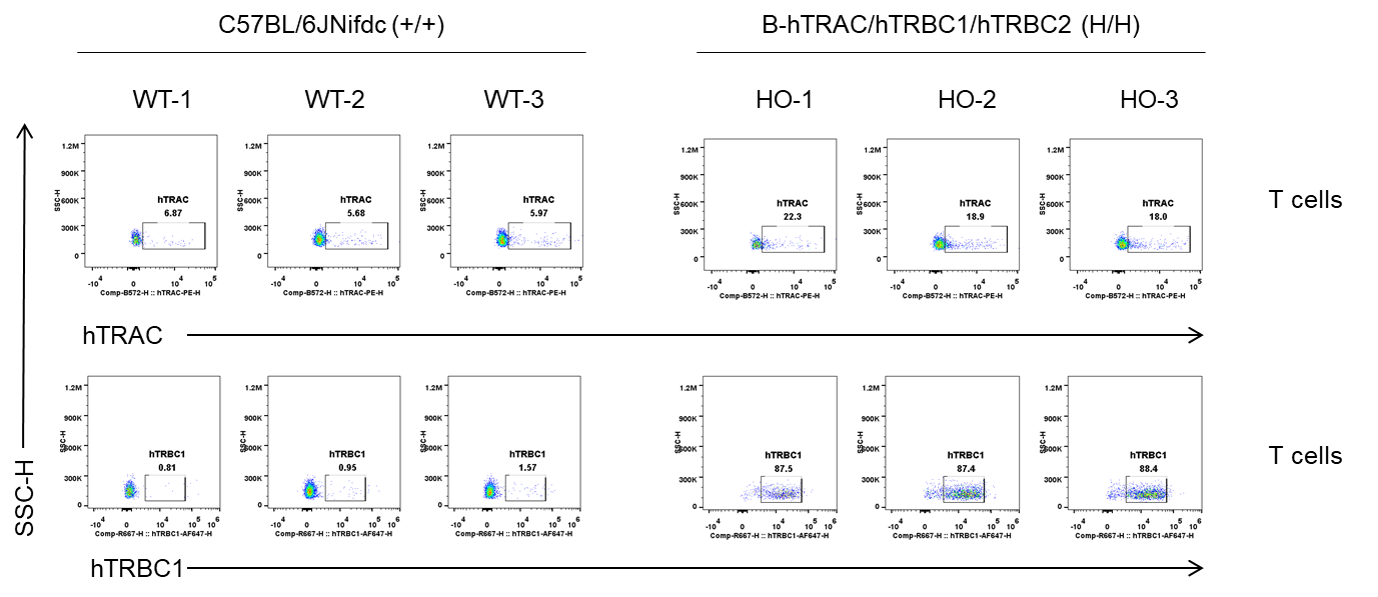
Strain specific TRAC and TRBC1 expression analysis in homozygous B-hTRAC/hTRBC1/hTRBC2 mice by flow cytometry. Blood cells were collected from wild-type C57BL/6JNifdc (+/+) and homozygous B-hTRAC/hTRBC1/hTRBC2 mice (H/H), and analyzed by flow cytometry with species-specific anti-human TRAC antibody (Invitrogen, PA5-95587) and anti-human TRBC1 antibody (Biolegend, 383502). Human TRAC was detectable in B-hTRAC/hTRBC1/hTRBC2 mice and weakly detectable in wild-type C57BL/6JNifdc mice because the antibody cross-recognizes human and mouse TRAC. Human TRBC1 was exclusively detectable in homozygous B-hTRAC/hTRBC1/hTRBC2 mice.
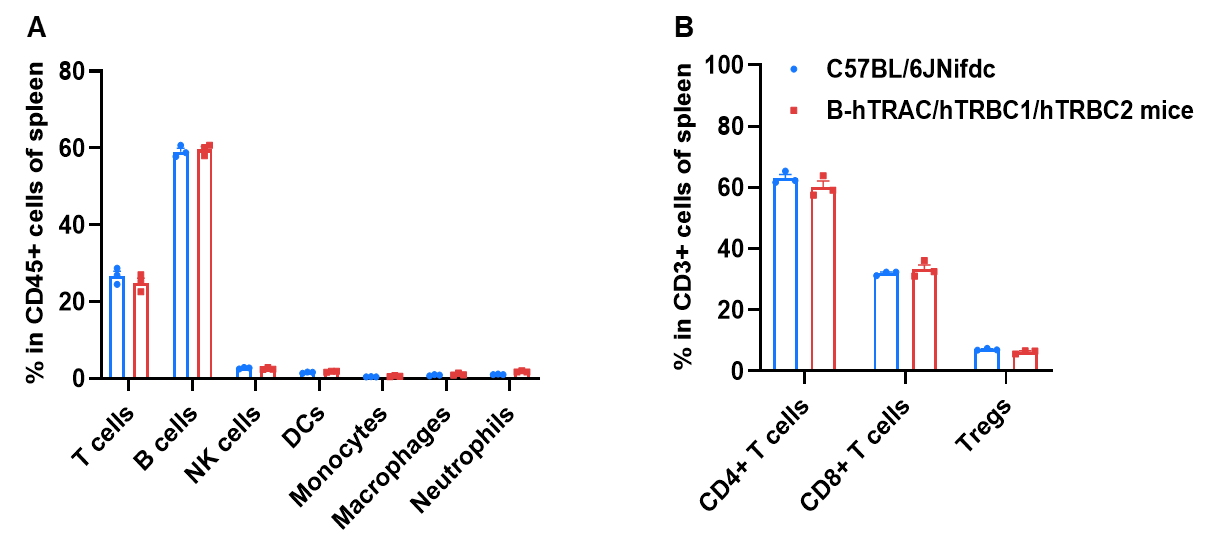
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6JNifdc mice and homozygous B-hTRAC/hTRBC1/hTRBC2 mice (male, 8-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Frequencies of T cells, B cells, NK cells, dendritic cells, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells, and Tregs in B-hTRAC/hTRBC1/hTRBC2 mice were similar to those in C57BL/6JNifdc mice, demonstrating that humanization of TRAC, TRBC1, and TRBC2 does not change the frequency or distribution of these cell types in spleen.
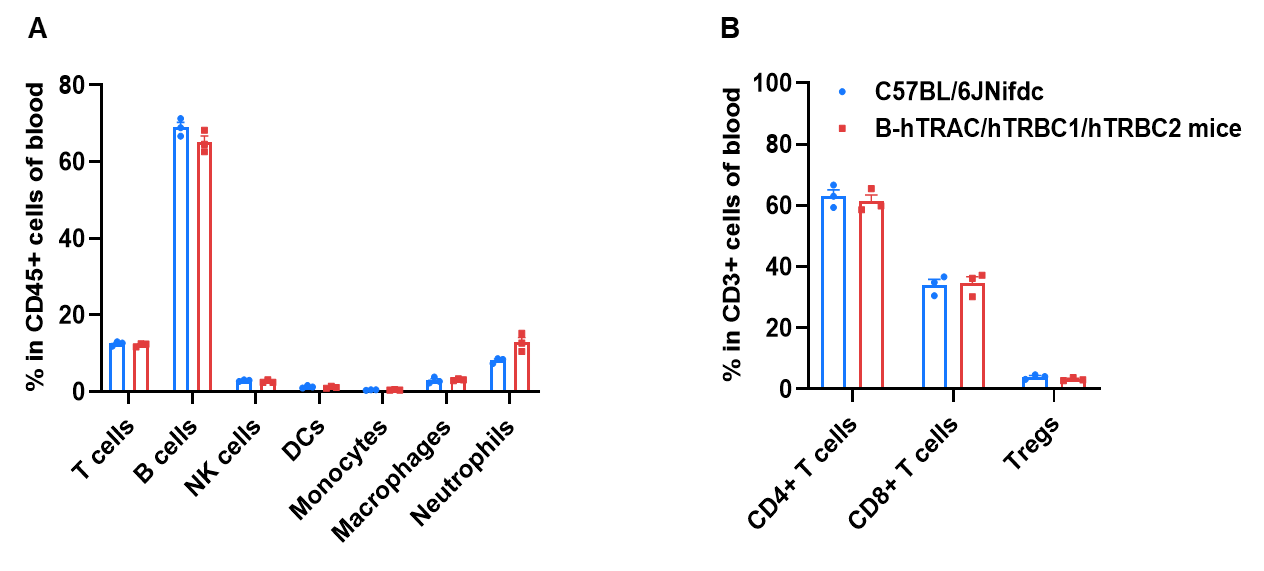
Frequency of leukocyte subpopulations in blood by flow cytometry. Blood cells were isolated from wild-type C57BL/6JNifdc mice and homozygous B-hTRAC/hTRBC1/hTRBC2 mice (male, 8-week-old, n=3). A. Flow cytometry analysis of the blood was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Frequencies of T cells, B cells, NK cells, dendritic cells, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells, and Tregs in B-hTRAC/hTRBC1/hTRBC2 mice were similar to those in C57BL/6JNifdc mice, demonstrating that humanization of TRAC, TRBC1, and TRBC2 does not change the frequency or distribution of these cell types in blood.
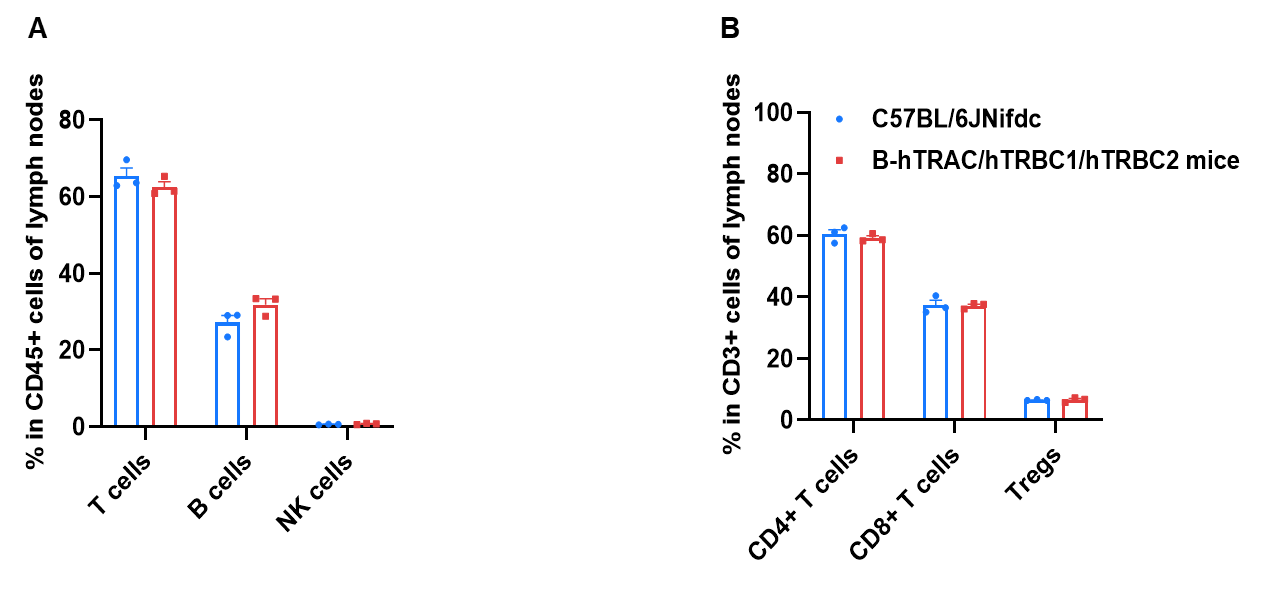
Frequency of leukocyte subpopulations in lymph node by flow cytometry. Lymph node cells were isolated from wild-type C57BL/6JNifdc mice and homozygous B-hTRAC/hTRBC1/hTRBC2 mice (male, 8-week-old, n=3). A. Flow cytometry analysis of the lymph node was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Frequencies of T cells, B cells, and NK cells in B-hTRAC/hTRBC1/hTRBC2 mice were similar to those in C57BL/6JNifdc mice, demonstrating that humanization of TRAC, TRBC1, and TRBC2 does not change the frequency or distribution of these cell types in lymph node.
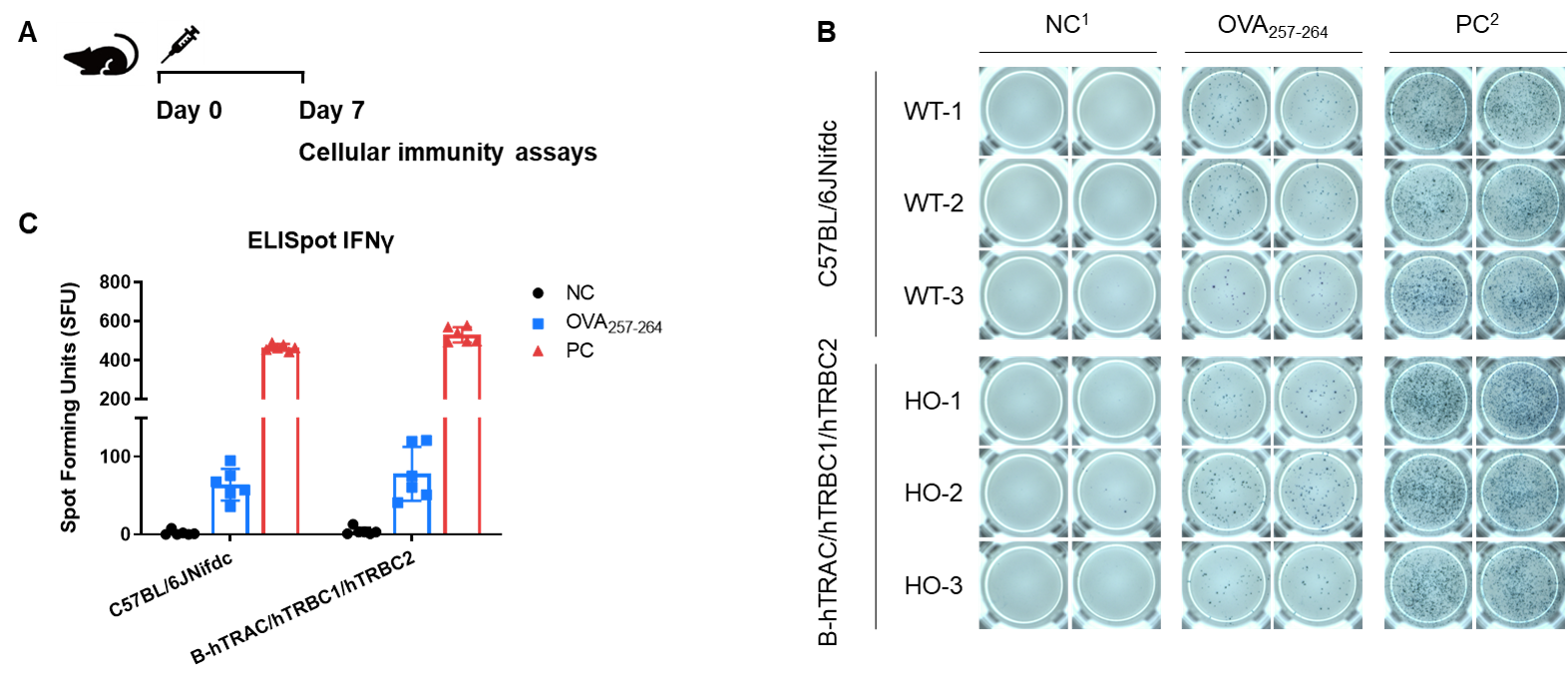
Detection of OVA-induced immune responses in B-hTRAC/hTRBC1/hTRBC2 mice by IFN-γ ELISpot assay. (A) Scheme of OVA immunization and testing. Female wild-type C57BL/6JNifdc mice and B-hTRAC/hTRBC1/hTRBC2 mice at the age of 9–10 weeks were immunized with intraperitoneal injection of 0.5 mg of OVA protein (Simga, A5503-25MG) and 50 μg poly (I:C) (InvivoGen, tlrl-pic). Mice were immunized with OVA one time. One week after the immunization, mice were sacrificed. The splenocytes were extracted, stimulated with OVA peptide257–264, or no peptide as negative control (NC), or Cell Activation Cocktail (without Brefeldin A), (Biolegend, 42330) as positive control, and then measured for IFN-γ secretion. No significant difference in body weight among groups (Data was not shown). (B) Representative results showing stimulation of splenocytes harvested from immunized mice with negative control, or OVA peptide257–264, or positive control in duplicates. (C) Summary of results. These data indicate that B-hTRAC/hTRBC1/hTRBC2 mice have normal T cell immunogenic function. 1, NC: negative control. 2, PC: positive control.
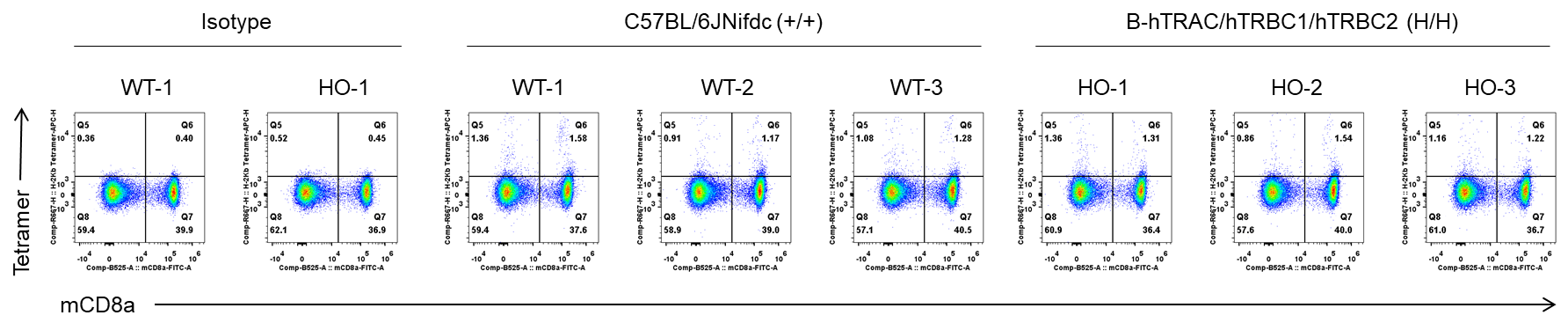
Frequency of OVA-specific T cells among spleen cells was assessed by flow cytometric analysis of SIINFEKL-MHC-I tetramer+ CD8+ T cells. Female wild-type C57BL/6JNifdc mice and B-hTRAC/hTRBC1/hTRBC2 mice at the age of 9–10 weeks were immunized with intraperitoneal injection of 0.5 mg of OVA protein (Simga, A5503-25MG) and 50 μg poly (I:C) (InvivoGen, tlrl-pic). Mice were immunized with OVA one time. One week after the immunization, mice were sacrificed and spleen cells stained with Tetramers(MBL, TS-5001-2C). OVA-specific T cells were detected. The value on the upper right of each plot indicates the percentage of Tetramer-positive CD8+ T cells. The frequencies of Tetramer-positive CD8+ T cells were similar between the wild-type C57BL/6JNifdc mice and B-hTRAC/hTRBC1/hTRBC2 mice.
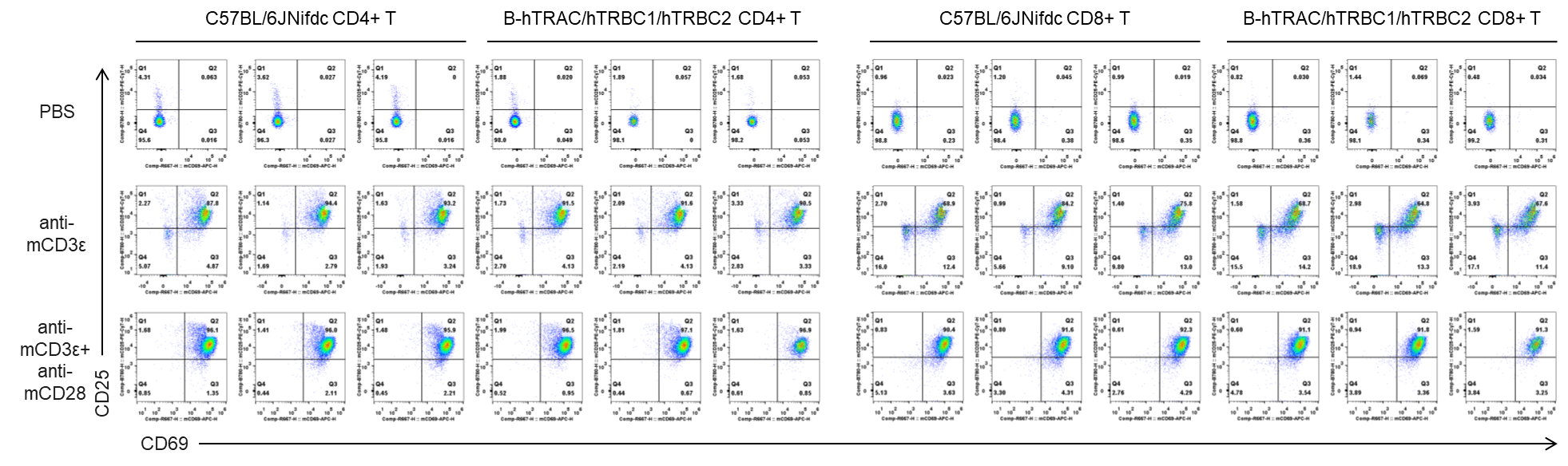
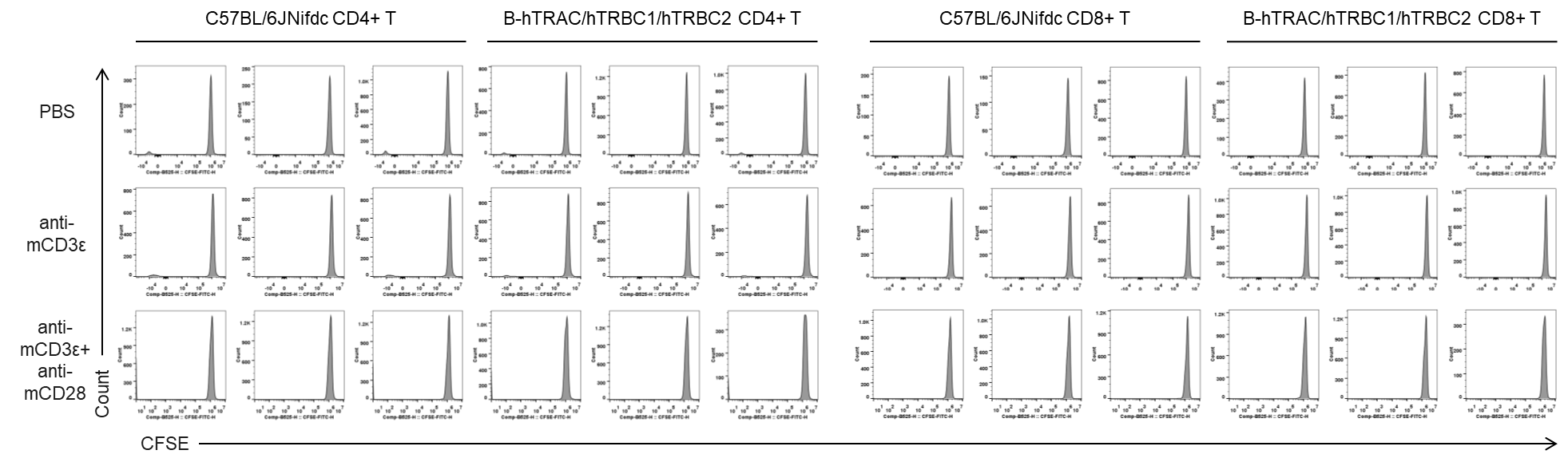
In vitro T cell activation by anti-mCD3ε antibody with or without anti-mCD28 antibody in wild-type C57BL/6JNifdc mice and homozygous humanized B-hTRAC/hTRBC1/hTRBC2 mice (24h). T cells were isolated from splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female,13-week-old, n=3), and incubated in the presence of anti-mCD3ε antibody (2ug/ml, BioXcell, BE0001-2), with or witnout anti-mCD28 antibody (5ug/ml, BioXcell, BE0015-1) for 24h. T cell proliferation was tested by flow cytometry. T cell activation in B-hTRAC/hTRBC1/hTRBC2 mice was significantly up-regulated by anti-mCD3ε antibody and anti-mCD28 antibody.
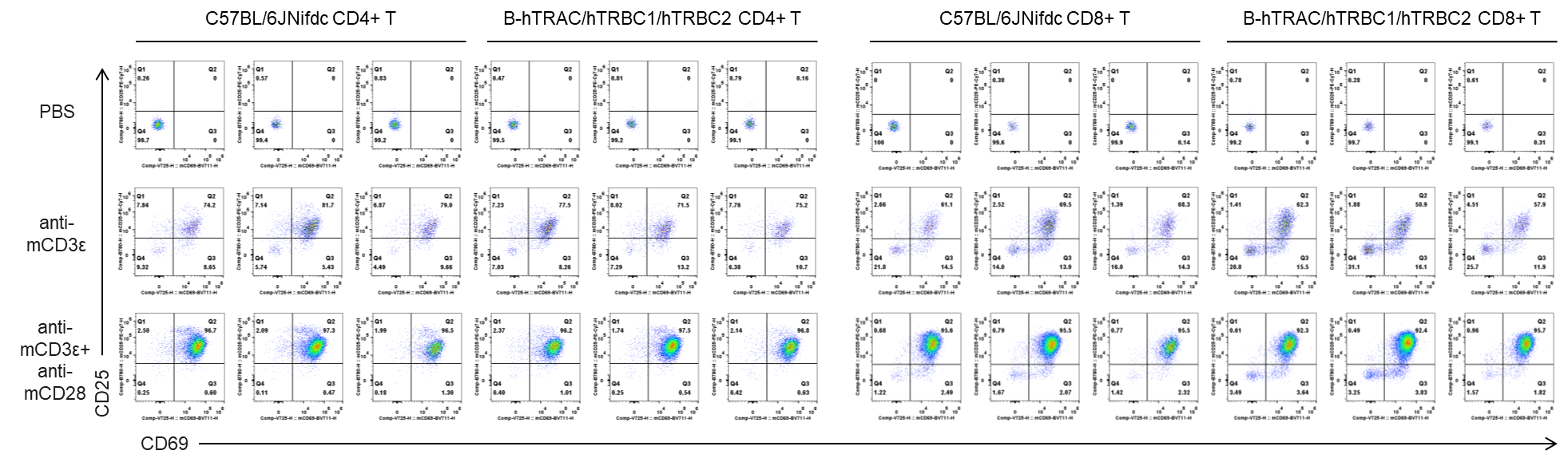
In vitro T cell activation by anti-mCD3ε antibody with or without anti-mCD28 antibody in wild-type C57BL/6JNifdc mice and homozygous humanized B-hTRAC/hTRBC1/hTRBC2 mice (48h). T cells were isolated from splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female,13-week-old, n=3), and incubated in the presence of anti-mCD3ε antibody (2ug/ml, BioXcell, BE0001-2), with or witnout anti-mCD28 antibody (5ug/ml, BioXcell, BE0015-1) for 24h. T cell proliferation was tested by flow cytometry. T cell activation in B-hTRAC/hTRBC1/hTRBC2 mice was significantly up-regulated by anti-mCD3ε antibody and anti-mCD28 antibody.
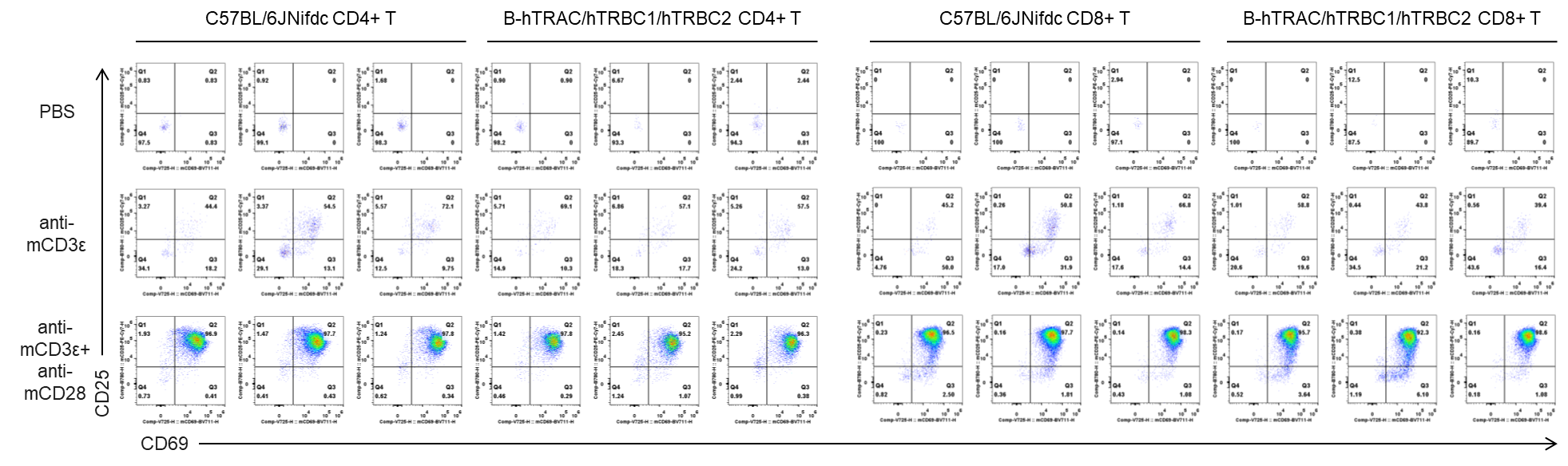
In vitro T cell activation by anti-mCD3ε antibody with or without anti-mCD28 antibody in wild-type C57BL/6JNifdc mice and homozygous humanized B-hTRAC/hTRBC1/hTRBC2 mice (72h). T cells were isolated from splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female,13-week-old, n=3), and incubated in the presence of anti-mCD3ε antibody (2ug/ml, BioXcell, BE0001-2), with or witnout anti-mCD28 antibody (5ug/ml, BioXcell, BE0015-1) for 24h. T cell proliferation was tested by flow cytometry. T cell activation in B-hTRAC/hTRBC1/hTRBC2 mice was significantly up-regulated by anti-mCD3ε antibody and anti-mCD28 antibody.
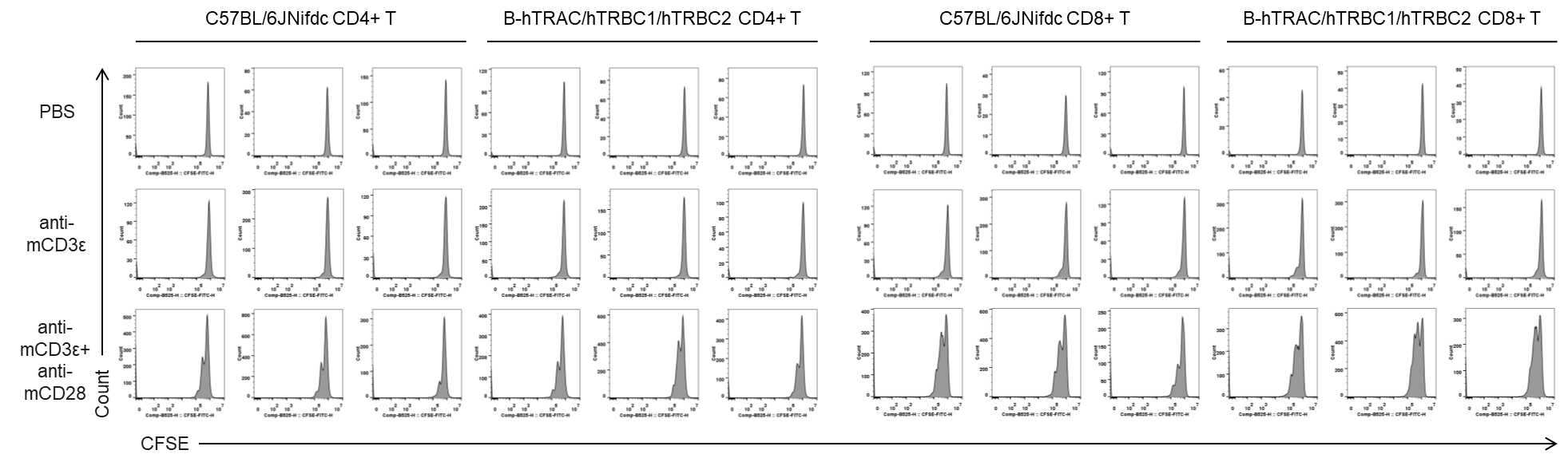
Quantification of T cell proliferation in vitro by anti-CD3ε antibody with or without anti-mCD28 antibody in wild-type C57BL/6JNifdc mice and homozygous humanized B-hTRAC/hTRBC1/hTRBC2 mice (24h). T cells were isolated from splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female,13-week-old, n=3), and incubated in the presence of anti-mCD3ε antibody (2ug/ml, BioXcell, BE0001-2), with or witnout anti-mCD28 antibody (5ug/ml, BioXcell, BE0015-1) for 24h. T cell proliferation was tested by flow cytometry.
Quantification of T cell proliferation in vitro by anti-CD3ε antibody with or without anti-mCD28 antibody in wild-type C57BL/6JNifdc mice and homozygous humanized B-hTRAC/hTRBC1/hTRBC2 mice (48h). T cells were isolated from splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female,13-week-old, n=3), and incubated in the presence of anti-mCD3ε antibody (2ug/ml, BioXcell, BE0001-2), with or witnout anti-mCD28 antibody (5ug/ml, BioXcell, BE0015-1) for 24h. T cell proliferation was tested by flow cytometry.
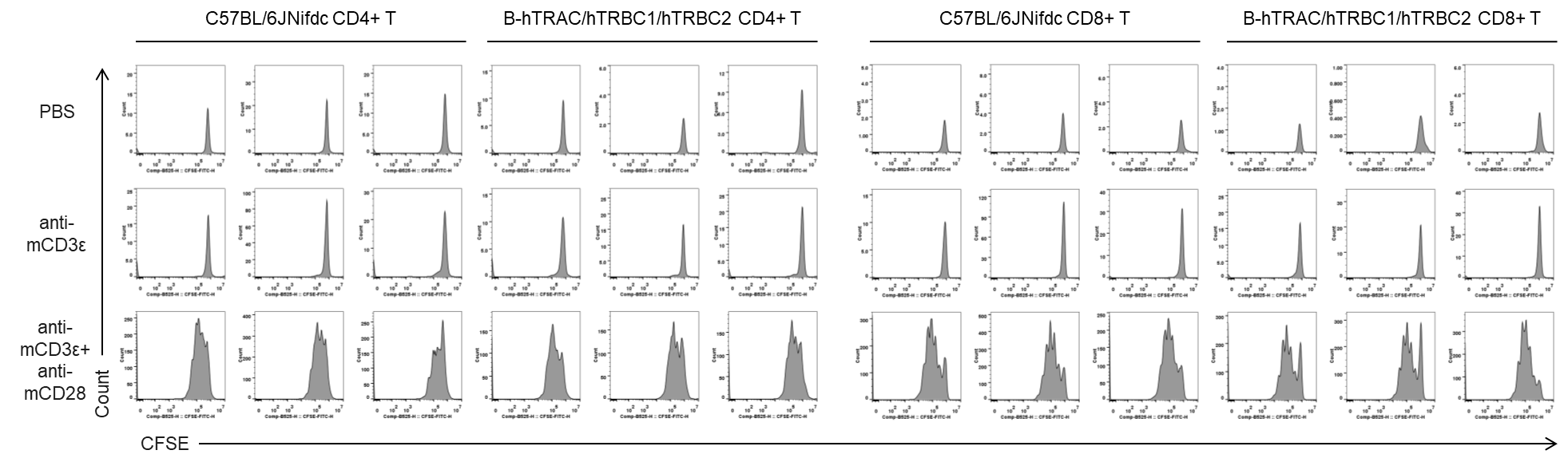
Quantification of T cell proliferation in vitro by anti-CD3ε antibody with or without anti-mCD28 antibody in wild-type C57BL/6JNifdc mice and homozygous humanized B-hTRAC/hTRBC1/hTRBC2 mice (72h). T cells were isolated from splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female,13-week-old, n=3), and incubated in the presence of anti-mCD3ε antibody (2ug/ml, BioXcell, BE0001-2), with or witnout anti-mCD28 antibody (5ug/ml, BioXcell, BE0015-1) for 24h. T cell proliferation was tested by flow cytometry.
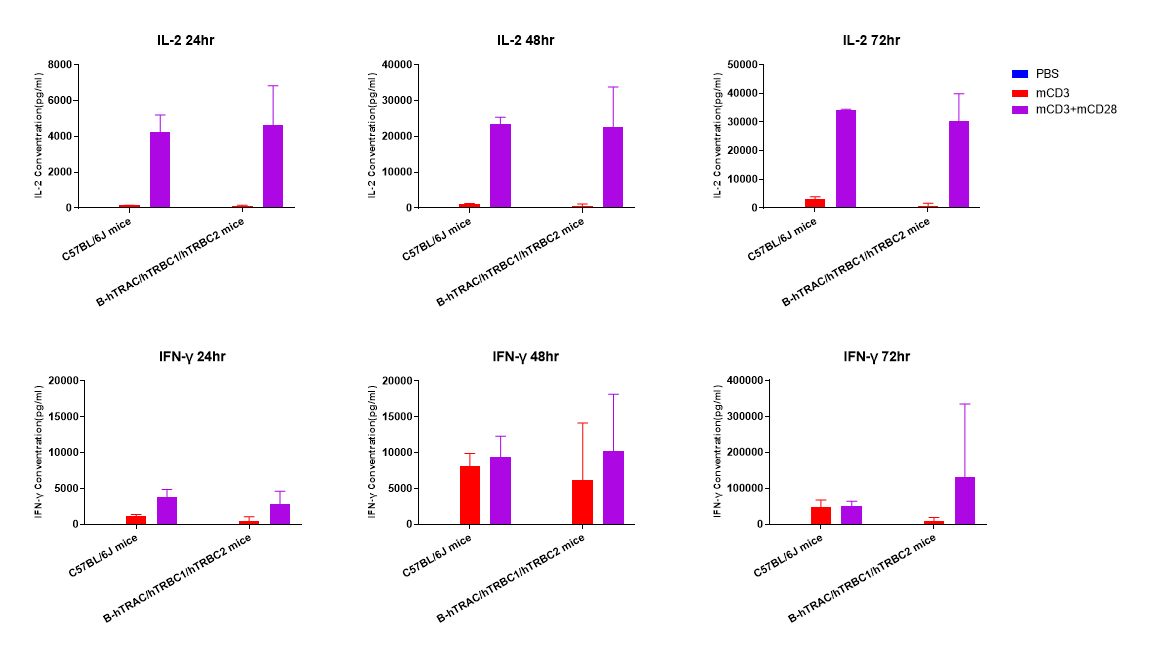
In vitro cytokine production (IFN-γ and IL-2) in B-hTRAC/hTRBC1/hTRBC2 humanized mice. T cells (2×105) were isolated from the splenocytes of C57BL/6JNifdc and B-hTRAC/hTRBC1/hTRBC2 mice (female, 13-week-old, n=3), incubated in the presence of anti-mouse CD3ε antibody (BioXCell, BE0001-1, clone 145-2C11, 2ug/ml) and anti-mCD28 antibody (BioXCell, BE0015-1, clone 37.51, 5ug/ml) for 24h, 48h and 72h. IFN-γ and IL-2 productions were then tested using ELISA method.
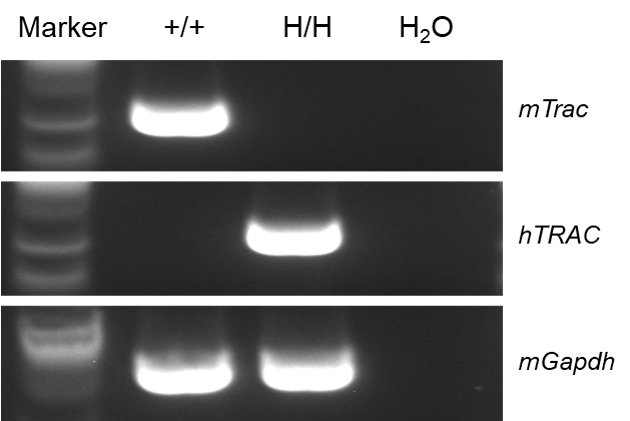
Species specific analysis of TRAC gene expression in wild-type C57BL/6JNifdc and homozygous B-hTRAC/hTRBC1/hTRBC2 mice by RT-PCR. The spleen tissues were collected from wild-type C57BL/6JNifdc (+/+) and homozygous B-hTRAC/hTRBC1/hTRBC2 mice (H/H). Mouse Trac mRNA was only detectable in wild-type C57BL/6JNifdc. Human TRAC mRNA was only detectable in homozygous B-hTRAC/hTRBC1/hTRBC2 mice, but not in wild-type C57BL/6JNifdc.