
C57BL/6-Tfrctm1(TFRC)Bcgen Mapttm1(MAPT)Bcgen/Bcgen • 113290

| Product name | B-hTFR1/hTAU mice |
|---|---|
| Catalog number | 113290 |
| Strain name | C57BL/6-Tfrctm1(TFRC)Bcgen Mapttm1(MAPT)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 7037,4137 (Human) |
| Aliases | T9; TR; TFR; p90; CD71; TFR1; TRFR; IMD46; TAU; FTD1; MSTD; PPND; DDPAC; MAPTL; MTBT1; MTBT2; tau-40; FTDP-17; PPP1R103; Tau-PHF6 |
| Application | This product is used for pharmacodynamics evaluation of Alzheimer's disease (AD). |
TFR1 (transferrin receptor 1) plays a critical role in iron transport across cell membranes and is highly expressed in brain endothelial cells, making it an attractive target for drug delivery across the blood–brain barrier. Humanized TAU, encoded by MAPT, is a key pathogenic factor in tauopathies, including Alzheimer's disease. By co-expressing human TFR1 and MAPT, this model enables integrated investigation of neurodegenerative disease mechanisms and the development of translational CNS therapeutic strategies.
TFR1/TAU humanized mice are genetically engineered mouse models in which both the Tfr1 and Mapt loci are humanized. In this model, exons 4–19 of the mouse Tfr1 gene, which encode the extracellular region responsible for receptor function, are replaced with the corresponding human TFR1 exons.
Simultaneously, exons 2–10 of the mouse Mapt gene are replaced with human MAPT exons 2–15, and the mouse 3′ UTR is substituted with its human counterpart, ensuring physiological expression of human TAU protein. Human MAPT expression is driven by the endogenous mouse promoter, while endogenous mouse Mapt expression is disrupted.
Key Advantages
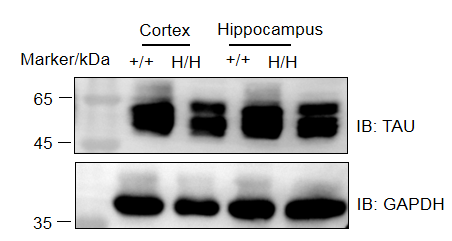
In B-hTFR1/hTAU mice, exons 2–10 of the mouse Mapt gene, which encode the full-length protein, are replaced with human MAPT exons 2–15. In addition, the 3′ UTR of the mouse Mapt gene is replaced with its human counterpart. Human MAPT expression is driven by the endogenous mouse Mapt promoter, while transcription and translation of the endogenous mouse Mapt gene are disrupted.
Simultaneously, exons 4–19 of the mouse Tfr1 gene, which encode the extracellular domain, are replaced with human TFR1 exons 4–19 in B-hTFR1/hTAU mice.
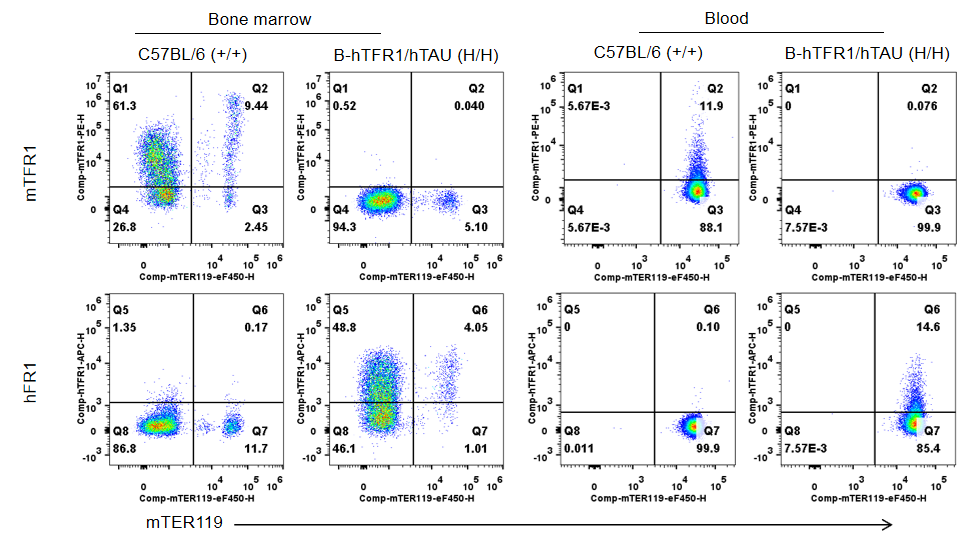
Strain-specific TFR1 expression analysis was performed by flow cytometry in homozygous TFR1/TAU humanized mice. Bone marrow and blood cells were collected from wild-type C57BL/6 mice (+/+) and homozygous TFR1/TAU humanized mice (H/H) and analyzed using anti-mouse TFR1 antibody (BioLegend, 113808) and anti-human TFR1 antibody (BioLegend, 334108). Mouse TFR1 was detectable in wild-type mice, whereas human TFR1 was exclusively detectable in homozygous TFR1/TAU humanized mice and not in wild-type mice.
Strain-specific TFR1 expression analysis was independently performed by flow cytometry in homozygous TFR1/TAU humanized mice. Bone marrow and blood cells were collected from wild-type C57BL/6 mice (+/+) and homozygous TFR1/TAU humanized mice (H/H) and analyzed using anti-mouse TFR1 antibody (BioLegend, 113808) and anti-human TFR1 antibody (BioLegend, 334108). Mouse TFR1 was detectable in wild-type mice, whereas human TFR1 was exclusively detectable in homozygous TFR1/TAU humanized mice and not in wild-type mice.
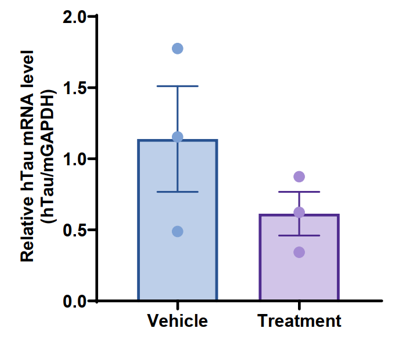
The inhibitory efficiency of drugs against human TAU was evaluated in TFR1/TAU humanized mice. This experiment was performed by the client using TFR1/TAU humanized mice, and all experimental materials were provided by the client. (Experimental details and results were supplied by the client.)
Q1: What are TFR1/TAU humanized mice?
A: TFR1/TAU humanized mice co-express human transferrin receptor 1 (TFR1) and human TAU protein (encoded by MAPT) under endogenous regulatory elements, enabling physiologically relevant in vivo modeling.
Q2: Why include human TFR1 in this model?
A: Human TFR1 is a key mediator of iron transport and is highly expressed in brain endothelial cells. It also serves as a receptor target for drug delivery across the blood–brain barrier, facilitating therapeutic access to the central nervous system.
Q3: What is the relevance of human TAU in this model?
A: Human TAU is centrally involved in tauopathies such as Alzheimer’s disease. Expression of human MAPT enables study of TAU biology and evaluation of MAPT-targeted therapies in a humanized context.
Q4: How can this model be used in therapeutic evaluation?
A: This model supports preclinical studies investigating neurodegenerative disease mechanisms, blood–brain barrier penetration strategies, and the pharmacodynamics of MAPT- and TFR1-targeted therapeutic agents.