
C57BL/6-Tnfrsf4tm1(TNFRSF4)Bcgen Tnfsf4tm1(TNFSF4)Bcgen/Bcgen • 120543

Key Advantages:
Application:
In the OX40/OX40L humanized mouse model, the exons 1–5 of the mouse Ox40 gene encoding the extracellular domain are replaced with human OX40 (TNFRSF4) exons 1–5. Similarly, the exons 2–3 of the mouse Ox40l gene encoding the extracellular region are replaced by human OX40L (TNFSF4) exons 2–3.
This targeting strategy ensures that human OX40 and human OX40L extracellular domains are expressed in place of their murine counterparts, enabling selective binding and in vivo evaluation of anti-human OX40/OX40L antibodies.
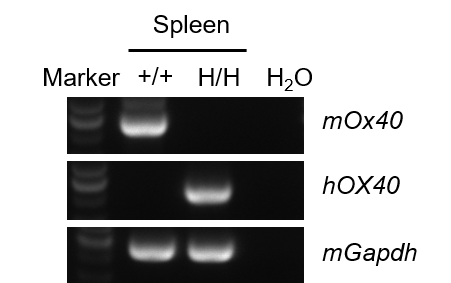
Strain-specific analysis of OX40 mRNA expression was performed in wild-type C57BL/6JNifdc mice and OX40/OX40L humanized mice using RT-PCR. Spleen RNA was isolated from wild-type C57BL/6JNifdc mice (+/+) and homozygous humanized OX40/OX40L mice (H/H; H/H). cDNA libraries were synthesized by reverse transcription, followed by PCR amplification using mouse- or human-specific OX40 primers. Mouse OX40 mRNA was detectable only in wild-type mice, whereas human OX40 mRNA was exclusively detected in homozygous OX40/OX40L humanized mice, confirming precise gene humanization of OX40.
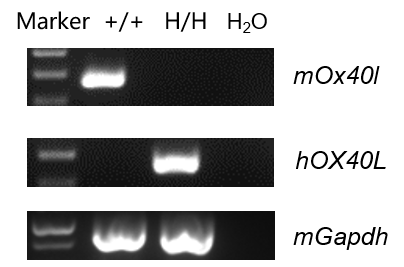
Strain-specific analysis of OX40L gene expression was performed by RT-PCR. Bone marrow cells were isolated from wild-type C57BL/6JNifdc mice (+/+) and homozygous humanized OX40/OX40L mice (H/H; H/H), followed by DC induction. Mouse Ox40l mRNA was detectable only in wild-type DCs, while human OX40L mRNA was detected exclusively in OX40/OX40L humanized mice, confirming successful replacement of the mouse Ox40l extracellular coding region with its human counterpart.
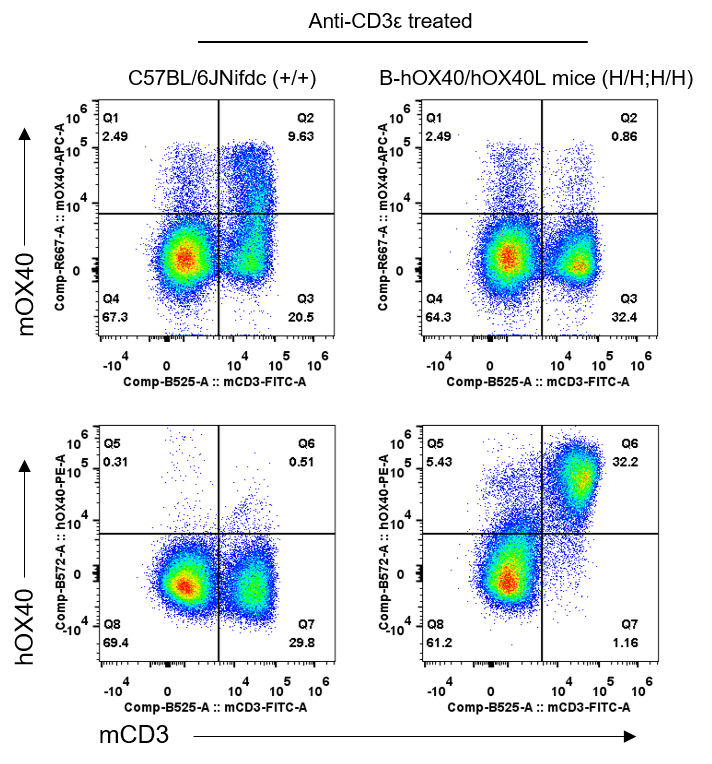
Human OX40 protein expression in OX40/OX40L humanized mice (flow cytometry). Splenocytes were isolated from wild-type C57BL/6JNifdc (+/+) and homozygous OX40/OX40L humanized mice (H/H; H/H) after in vivo anti-CD3ε stimulation. Protein expression was examined using anti-mouse OX40 (BioLegend, 119414) and anti-human OX40 (BioLegend, 350004) antibodies. Mouse OX40 protein was detectable only in T cells from wild-type mice, while human OX40 protein was detected exclusively in T cells from OX40/OX40L humanized mice, confirming functional human OX40 expression.
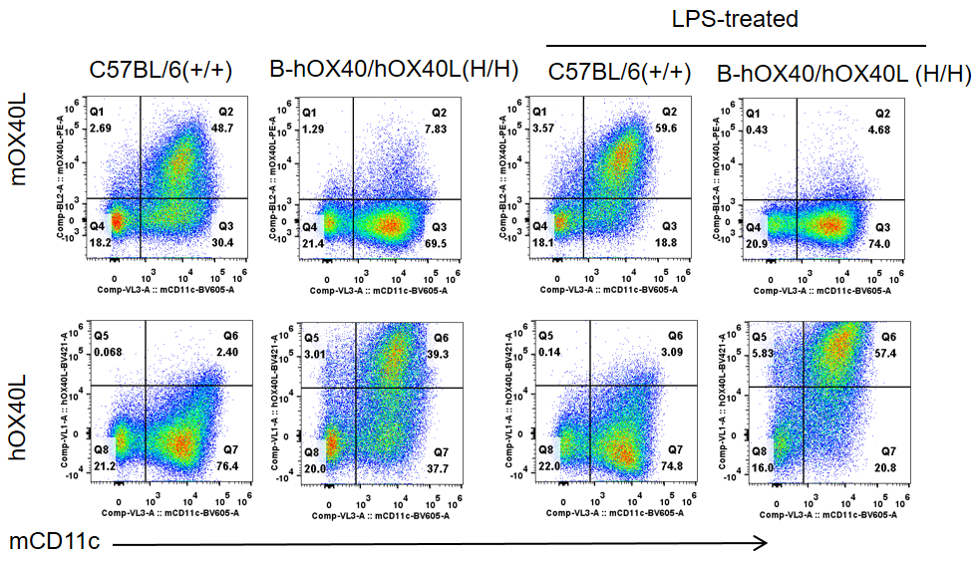
Human OX40L protein expression in OX40/OX40L humanized mice (flow cytometry). Bone marrow cells were collected from wild-type and OX40/OX40L humanized mice, differentiated into DCs, and stimulated with LPS. DCs were analyzed using anti-OX40L antibodies. Mouse OX40L was detectable only in wild-type samples, whereas human OX40L protein was exclusively detected in DCs from OX40/OX40L humanized mice, supporting correct surface expression of the humanized ligand.
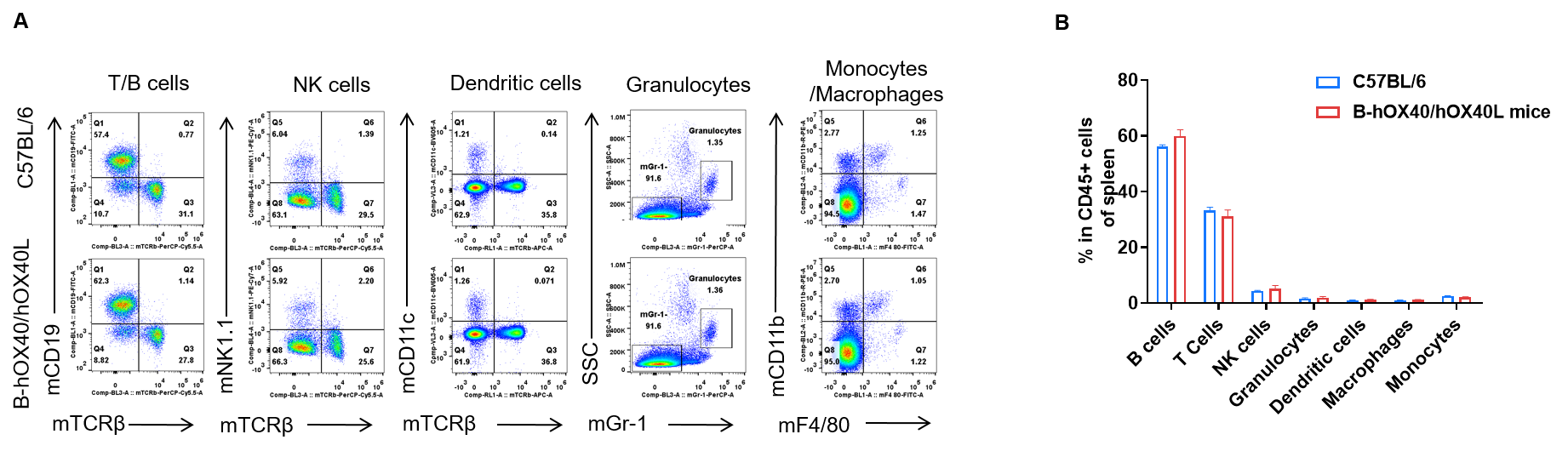
Spleen leukocyte subpopulation analysis by FACS in OX40/OX40L humanized mice. Analysis of spleen leukocyte subpopulations was performed by FACS. Splenocytes were isolated from female C57BL/6 and OX40/OX40L humanized mice (n = 3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots: single live cells were gated on the CD45⁺ population and used for further analysis as indicated. (B) Results of FACS analysis: the percentages of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous OX40/OX40L humanized mice were similar to those in C57BL/6 mice, demonstrating that introduction of human OX40/OX40L in place of the mouse counterparts does not change the overall development, differentiation or distribution of these spleen leukocyte populations. Values are expressed as mean ± SEM.
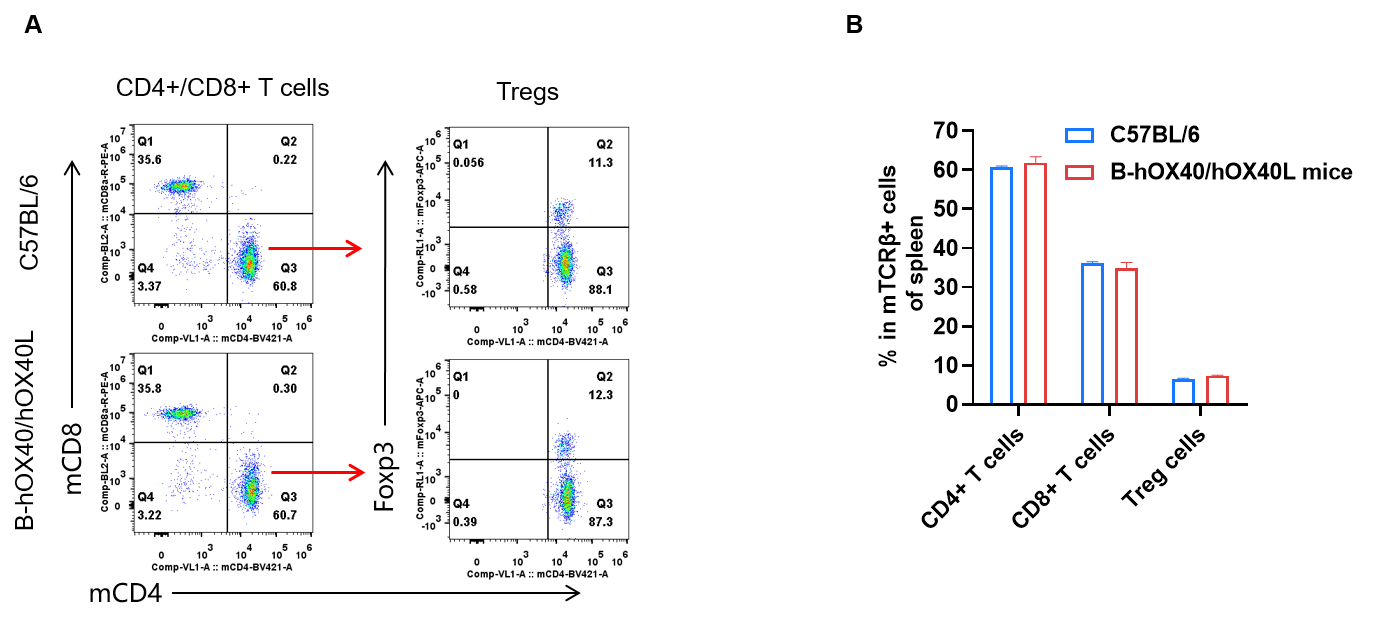
Spleen T-cell subpopulation analysis by FACS in humanized OX40/OX40L mice. Analysis of spleen T-cell subpopulations was performed by FACS. Splenocytes were isolated from female C57BL/6 and OX40/OX40L humanized mice (n = 3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess T-cell subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated on the TCRβ⁺ T-cell population and used for further analysis as indicated. (B) Results of FACS analysis: the percentages of CD8⁺ T cells, CD4⁺ T cells and Tregs in homozygous OX40/OX40L humanized mice were similar to those in C57BL/6 mice, demonstrating that introduction of human OX40/OX40L in place of its mouse counterpart does not alter the overall development, differentiation or distribution of T-cell subsets in spleen. Values are expressed as mean ± SEM.
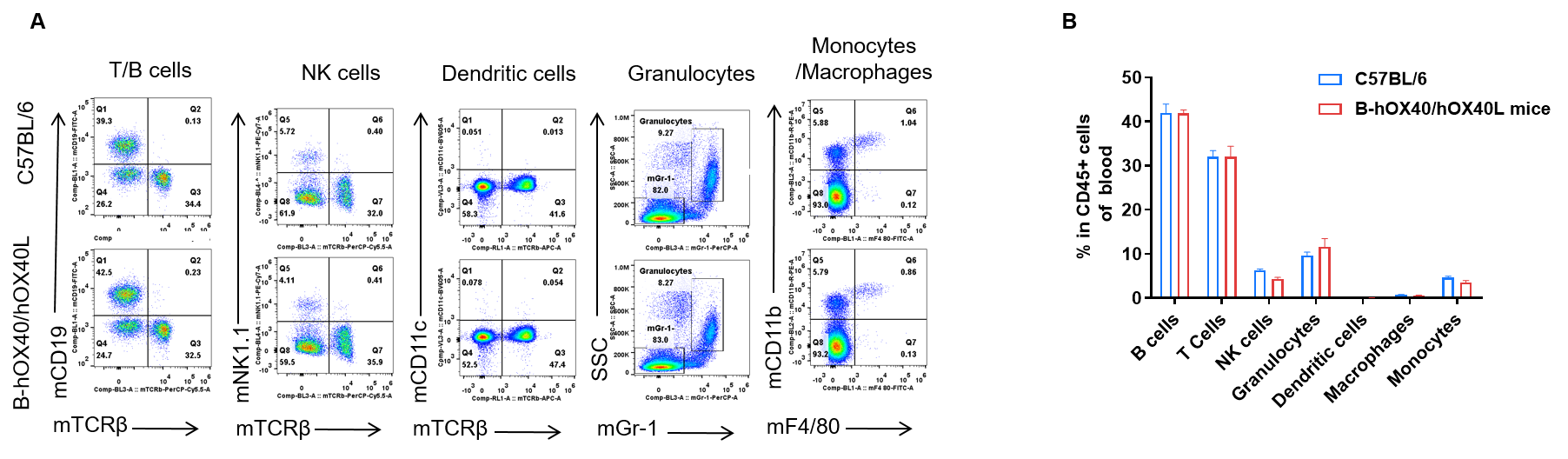
Blood leukocyte subpopulation analysis by FACS in OX40/OX40L humanized mice. Analysis of blood leukocyte subpopulations was performed by FACS. Blood cells were isolated from female C57BL/6 and OX40/OX40L humanized mice (n = 5, 6-week-old). Flow cytometry analysis of the blood cells was performed to assess leukocyte subpopulations. (A) Representative FACS plots: single live cells were gated on the CD45⁺ population and used for further analysis as indicated. (B) Results of FACS analysis: the percentages of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous OX40/OX40L humanized mice were similar to those in C57BL/6 mice, demonstrating that introduction of human OX40/OX40L in place of its mouse counterpart does not change the overall development, differentiation or distribution of these blood leukocyte populations. Values are expressed as mean ± SEM.
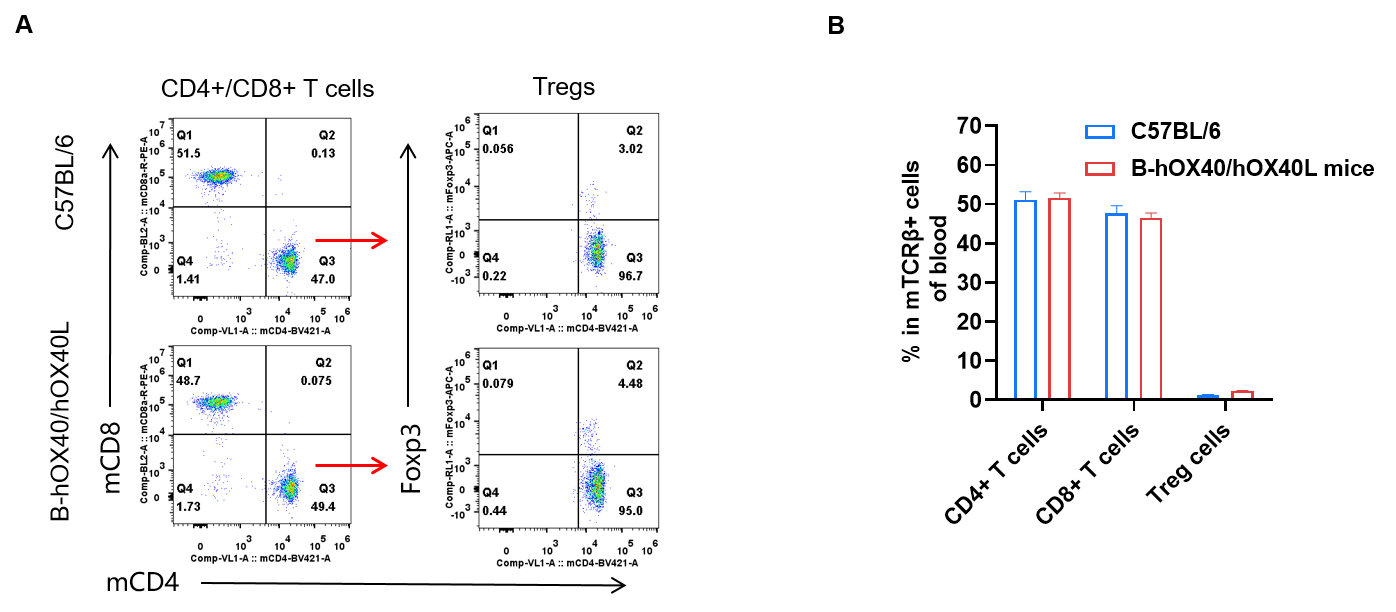
Blood T-cell subpopulation analysis by FACS in OX40/OX40L humanized mice. Analysis of blood T-cell subpopulations was performed by FACS. Blood cells were isolated from female C57BL/6 and OX40/OX40L humanized mice (n = 3, 6-week-old). Flow cytometry analysis of the blood was performed to assess T-cell subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated on the TCRβ⁺ T-cell population and used for further analysis as indicated. (B) Results of FACS analysis: the percentages of CD8⁺ T cells, CD4⁺ T cells and Tregs in homozygous OX40/OX40L humanized mice were similar to those in C57BL/6 mice, demonstrating that introduction of human OX40/OX40L in place of its mouse counterpart does not change the overall development, differentiation or distribution of T-cell subsets in blood. Values are expressed as mean ± SEM.
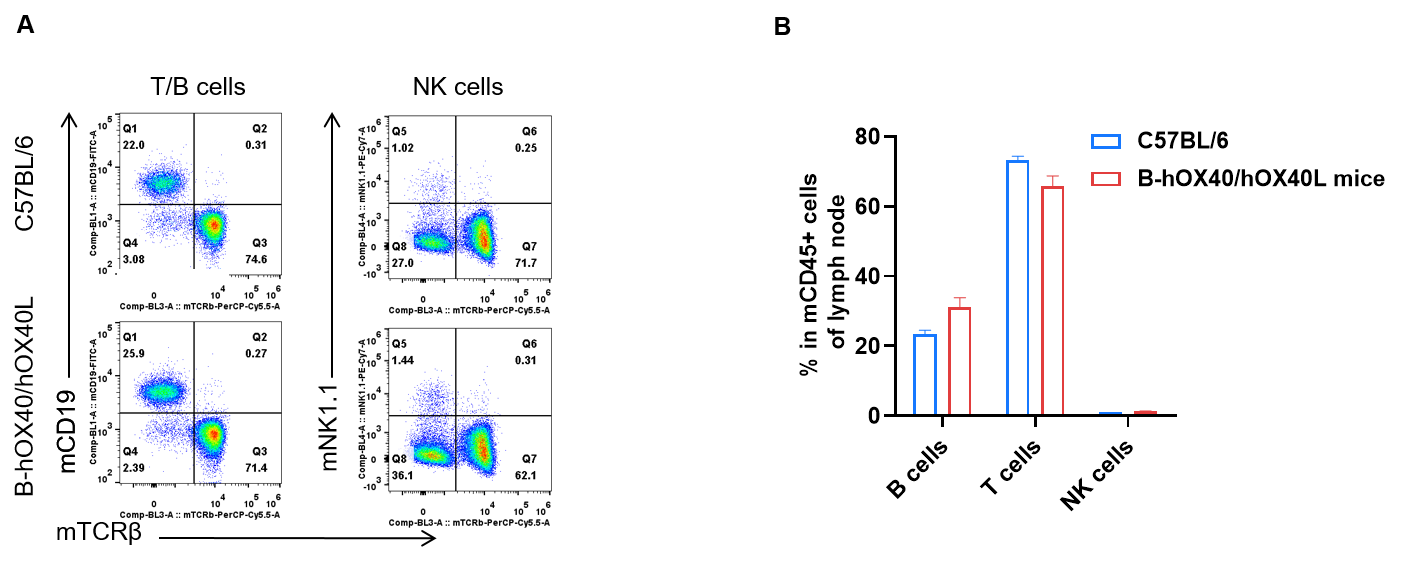
Lymph node leukocyte subpopulation analysis by FACS in OX40/OX40L humanized mice. Analysis of lymph node leukocyte subpopulations was performed by FACS. Lymph nodes were isolated from female C57BL/6 and OX40/OX40L humanized mice (n = 3, 6-week-old). Flow cytometry analysis of lymph node leukocytes was performed to assess leukocyte subpopulations. (A) Representative FACS plots: single live cells were gated on the CD45⁺ population and used for further analysis as indicated. (B) Results of FACS analysis: the percentages of T cells, B cells and NK cells in homozygous OX40/OX40L humanized mice were similar to those in C57BL/6 mice, demonstrating that introduction of human OX40/OX40L does not change the development, differentiation or distribution of these lymph node leukocyte populations. Values are expressed as mean ± SEM.
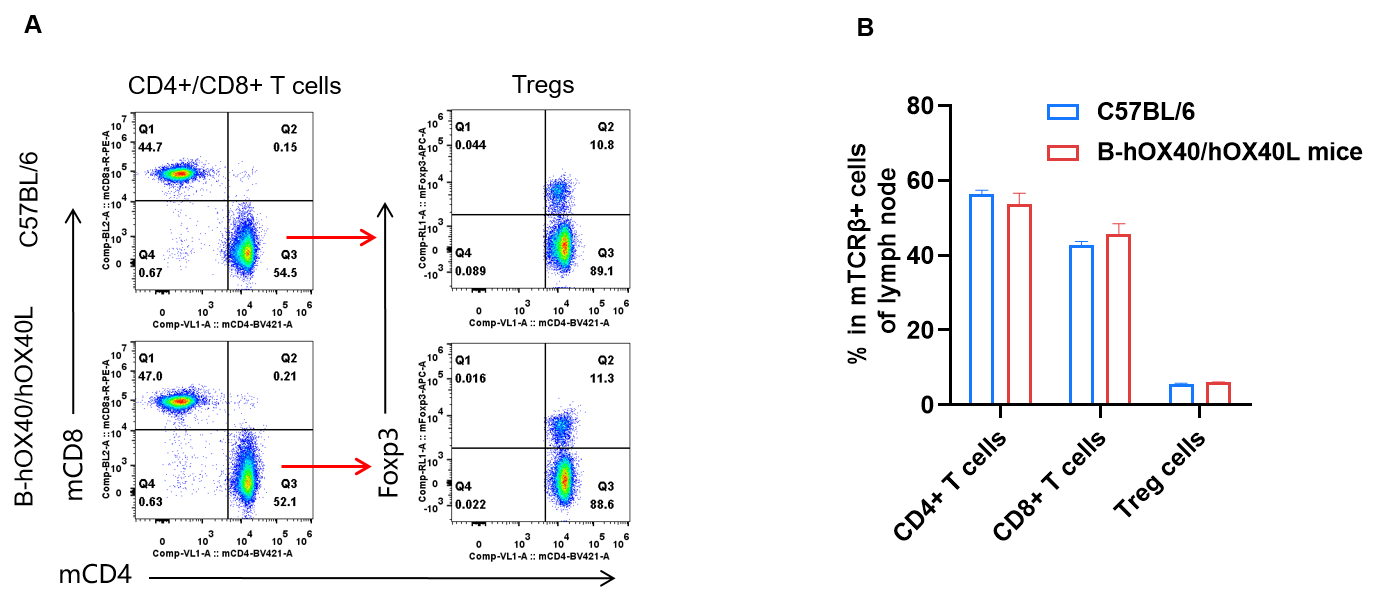
Analysis of lymph node T-cell subpopulations was performed by FACS. Lymph nodes were isolated from female C57BL/6 and OX40/OX40L humanized mice (n = 3, 6-week-old). Flow cytometry analysis of the leukocytes was performed to assess T-cell subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated on the TCRβ⁺ T-cell population and used for further analysis as indicated. (B) Results of FACS analysis: the percentages of CD8⁺ T cells, CD4⁺ T cells and Tregs in homozygous OX40/OX40L humanized mice were similar to those in C57BL/6 mice, demonstrating that humanization of OX40/OX40L does not change the overall development, differentiation or distribution of T-cell subsets in lymph nodes. Values are expressed as mean ± SEM.
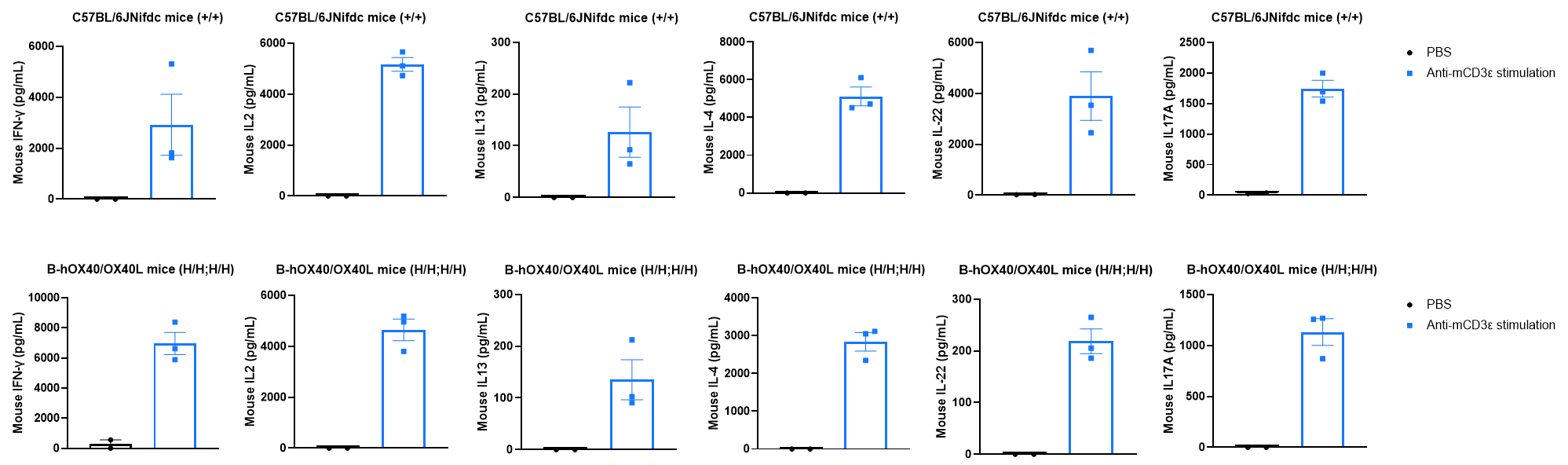
Cytokine expression in wild-type and OX40/OX40L humanized mice by ELISA. Strain-specific cytokine expression analysis was performed in wild-type C57BL/6JNifdc mice and homozygous OX40/OX40L humanized mice by ELISA. Serum was collected from wild-type C57BL/6JNifdc mice (+/+) (female, n = 3, 7-week-old) and homozygous OX40/OX40L humanized mice (H/H; H/H) (female, n = 3, 7-week-old) stimulated with anti-mouse CD3ε antibody (37.5 μg/mL, 200 μL/mouse, i.p.) for 2 h in vivo. Expression levels of mouse IL-4, IL-13, IL-2, IL-22, IL-17A and IFN-γ were analyzed by ELISA. After mCD3ε stimulation, a significant increase in mouse IL-4, IL-13, IL-2, IL-22, IL-17A and IFN-γ was detected in both C57BL/6JNifdc mice (n = 3) and homozygous OX40/OX40L humanized mice (n = 3). Under mouse CD3ε stimulation, downstream cytokines in the humanized mice fully responded, indicating that the signaling pathway in OX40/OX40L humanized mice is functionally intact. Values are expressed as mean ± SEM.
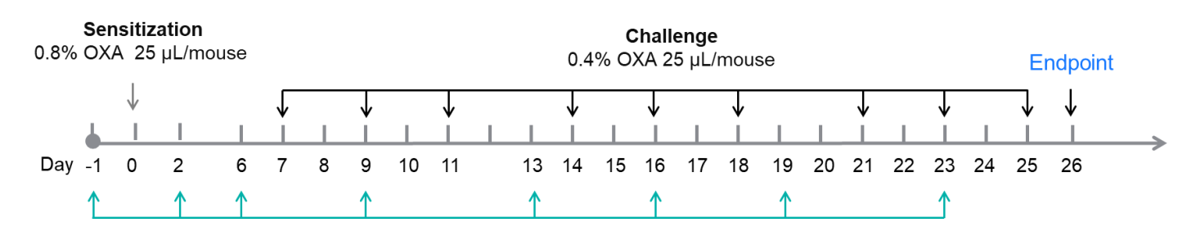
Experimental design for induction of AD-like skin lesions and anti-human OX40L antibody treatment. Experimental schedule for induction of AD-like skin lesions and in vivo efficacy of anti-human OX40L antibody in OX40/OX40L humanized mice. OXA was applied to dorsal and ear skin of mice on day 0, and then the same skin sites were challenged nine times from days 7 to 25. Anti-human OX40L antibody (provided by a client) was administered by intraperitoneal injection twice a week from days −1 to 23. Serum was collected at the endpoint on day 26. AD: atopic dermatitis; OXA: oxazolone.
Note: This experiment is a collaboration with the client.
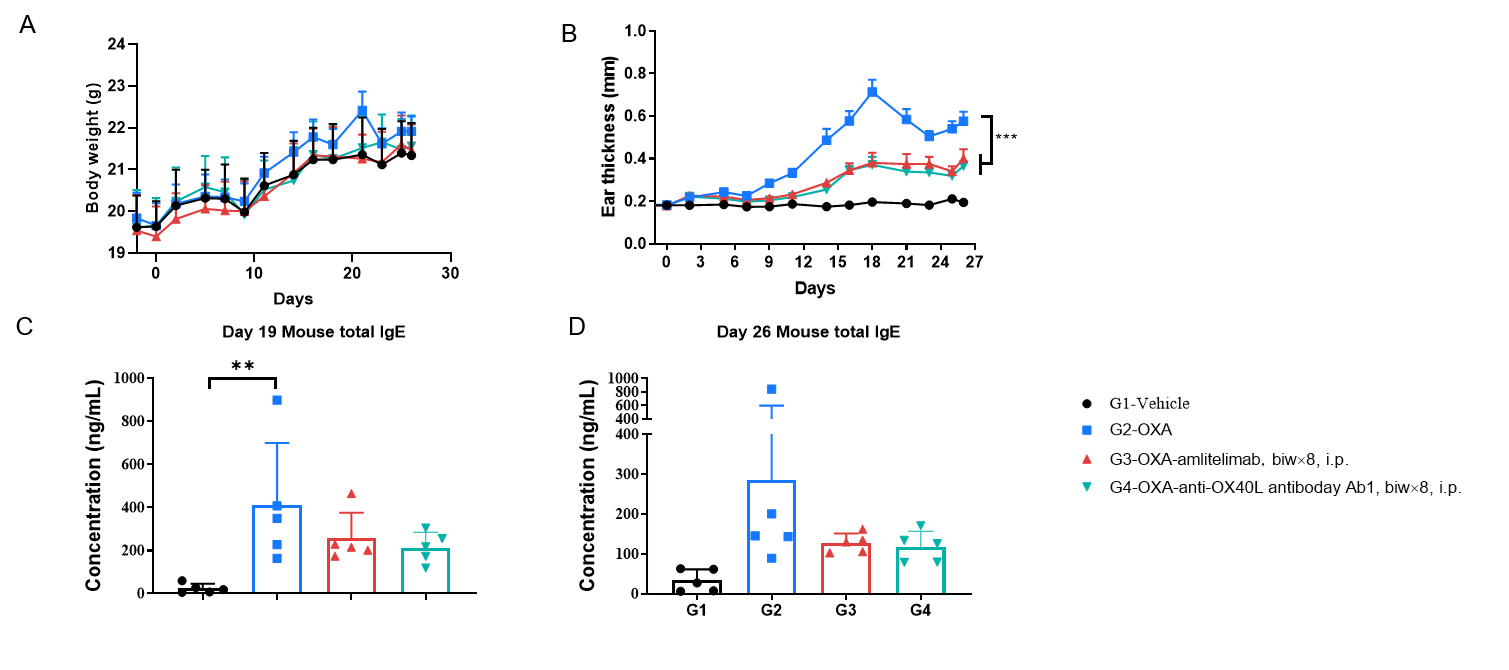
Efficacy of anti-human OX40L antibody was evaluated in OX40/OX40L humanized mice. Mice in each group were intraperitoneally injected with human OX40L antibody (provided by a client, n = 5). (A) Body weight changes during treatment. (B) Statistical analysis of ear thickness in each group. (C & D) Total IgE levels in serum. The results showed that, compared to the untreated group (G2), mice treated with anti-OX40L antibody showed a significant reduction in ear thickness. Serum was collected at the study endpoint, and IgE levels were analyzed by ELISA. The results showed that total IgE levels in mice treated with anti-OX40L antibody were lower than those in untreated mice. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
Note: This experiment is a collaboration with the client.
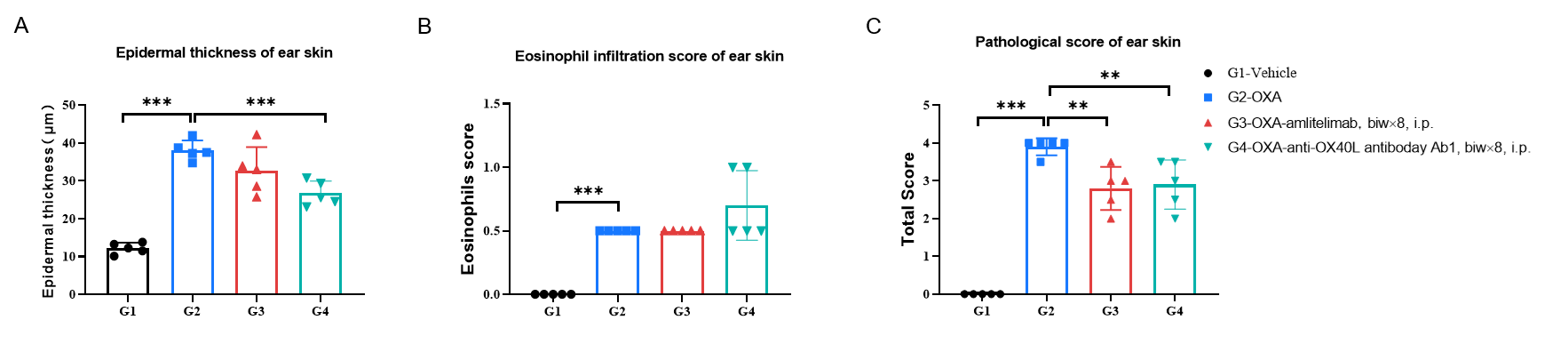
Efficacy of anti-human OX40L antibody in OX40/OX40L humanized mice was further evaluated by H&E staining. Ear tissues were collected at the study endpoint and analyzed histologically. The results showed that, compared to the untreated group (G2), mice treated with anti-OX40L antibody (provided by a client) showed a significant reduction in epidermal thickness and pathological scores of ear skin. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. AD: atopic dermatitis.
Note: This experiment is a collaboration with the client.
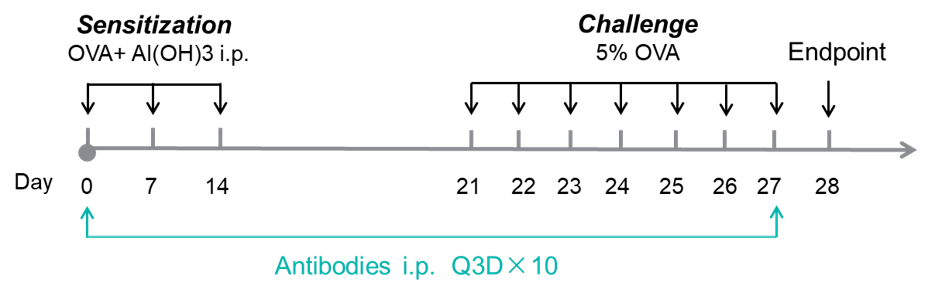
Experimental design for induction of asthma and anti-human OX40L antibody treatment. Experimental schedule for induction of asthma and in vivo efficacy of anti-human OX40L antibody in OX40/OX40L humanized mice. OVA + Al(OH)₃ was injected intraperitoneally on days 0, 7 and 14, followed by daily nebulization with OVA for the challenge phase from days 21 to 27. Anti-human OX40L antibody amlitelimab (in-house) was administered by intraperitoneal injection every 3 days from days 0 to 27. Serum was collected at the endpoint on day 28. OVA: ovalbumin.
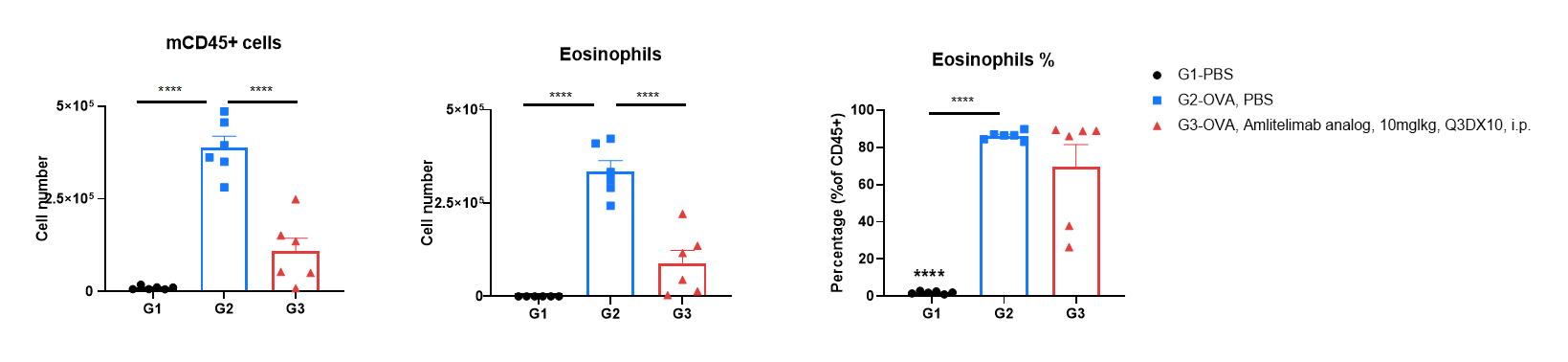
Analysis of immune cells in BALF was performed by flow cytometry. OX40/OX40L humanized mice (female, 7-week-old, n = 6) were immunized with OVA to induce asthma. Anti-human OX40L antibody (amlitelimab analog, synthesized in-house) was intraperitoneally injected from day 0 to day 27. Bronchoalveolar lavage fluid (BALF) was collected at the end of the experiment to detect inflammatory cell infiltration in lung tissue. The results showed that the number of CD45⁺ cells and eosinophils in BALF in the amlitelimab-treated group (G3) decreased significantly compared with the OVA-induced untreated group (G2). These data indicate that anti-human OX40L antibodies can effectively reduce the number and proportion of eosinophils in OVA-induced OX40/OX40L humanized OX40L mice. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
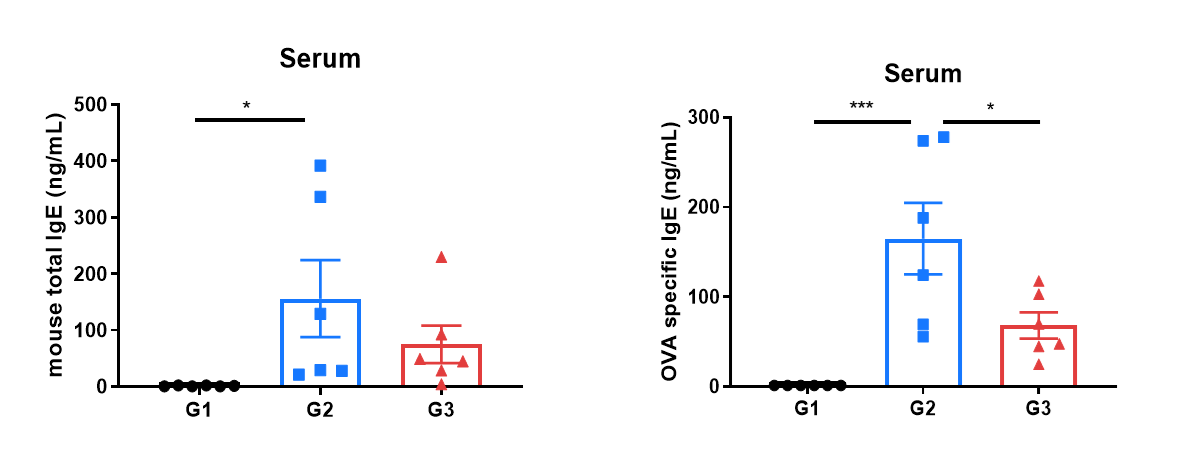
Mouse total IgE and OVA-specific IgE in serum were reduced in the asthma model treated with anti-OX40L antibody. Serum was collected at the study endpoint, and IgE levels were analyzed by ELISA. The results showed that the level of OVA-specific IgE in mice treated with amlitelimab (in-house) was lower than that in untreated mice. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
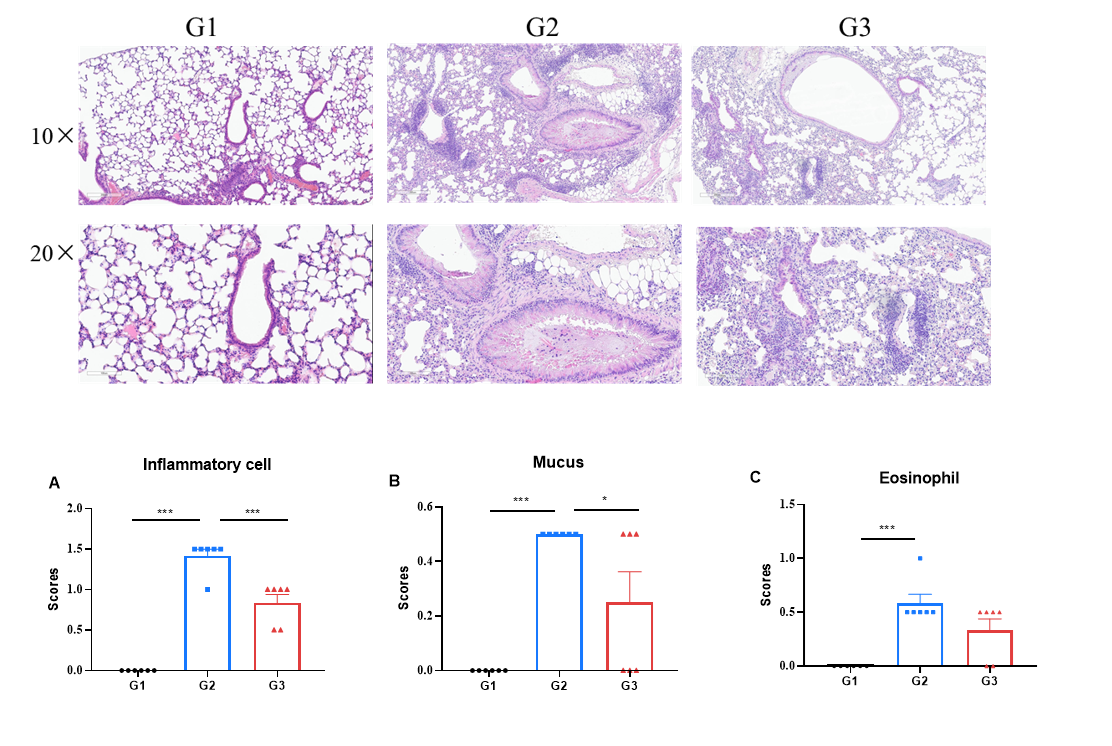
H&E staining was performed on lung tissues from the asthma-like model in OX40/OX40L humanized mice. Lung tissues were collected at the study endpoint and analyzed by H&E staining. The results showed that, compared to the untreated group (G2), mice treated with amlitelimab (in-house) showed a significant reduction in inflammatory infiltration and mucus secretion in lung tissue, indicating that OX40/OX40L humanized mice provide a powerful preclinical asthma model for in vivo evaluation of anti-human OX40L antibodies. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Efficacy of anti-human OX40L antibody was evaluated in an AD model using OX40/OX40L humanized mice. (A & B) Ear thickness and body weight changes during treatment. (C) Total IgE levels in serum. The results showed that, compared to the untreated group (G2), mice treated with amlitelimab (in-house) showed a significant reduction in ear thickness. Serum was collected at the study endpoint, and IgE levels were analyzed by ELISA. The results showed that total IgE levels in mice treated with amlitelimab (in-house) were lower than those in untreated mice. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
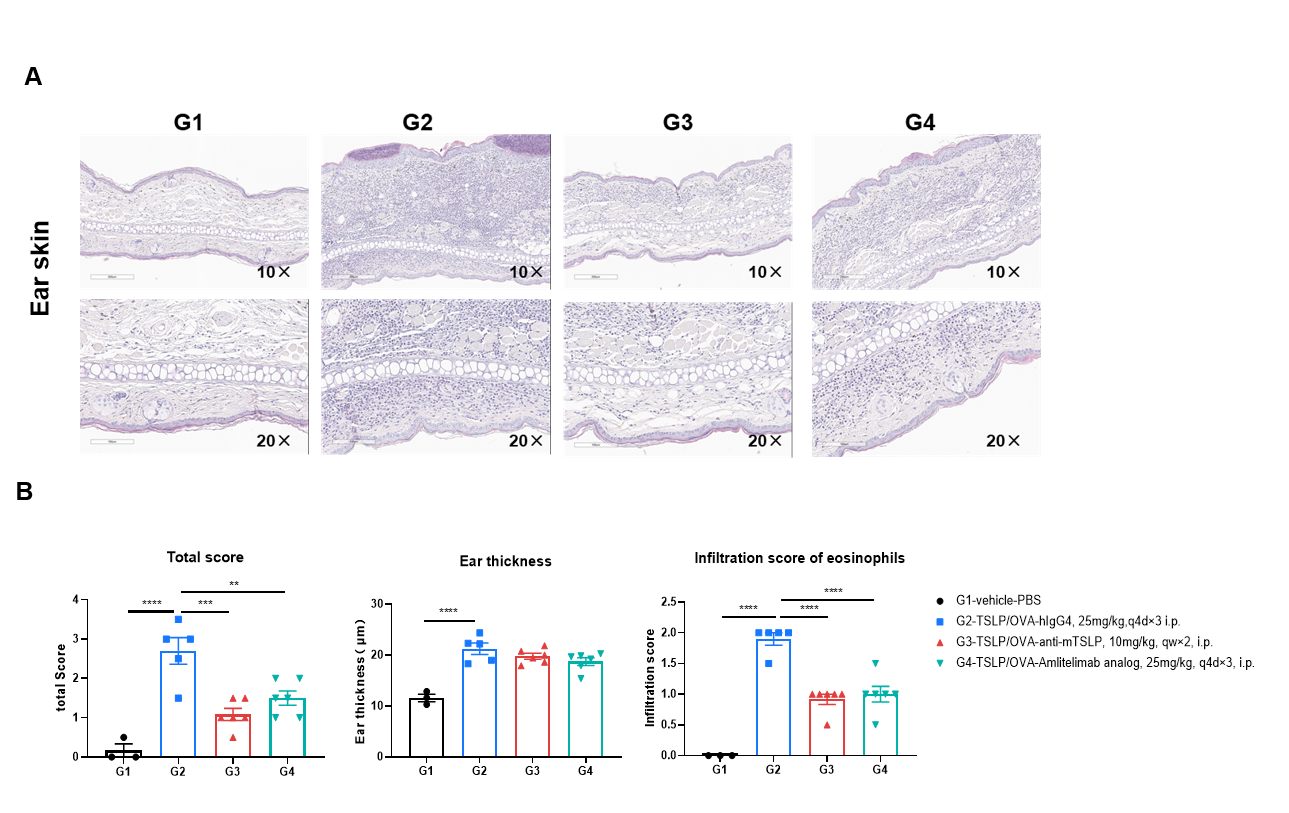
H&E staining of the AD-like model in OX40/OX40L humanized mice. Ear tissues were collected at the study endpoint and analyzed by H&E staining. The results showed that, compared to the untreated group (G2), mice treated with amlitelimab (in-house) showed a significant reduction in histopathology scores and scores of eosinophil infiltration. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. AD: atopic dermatitis.
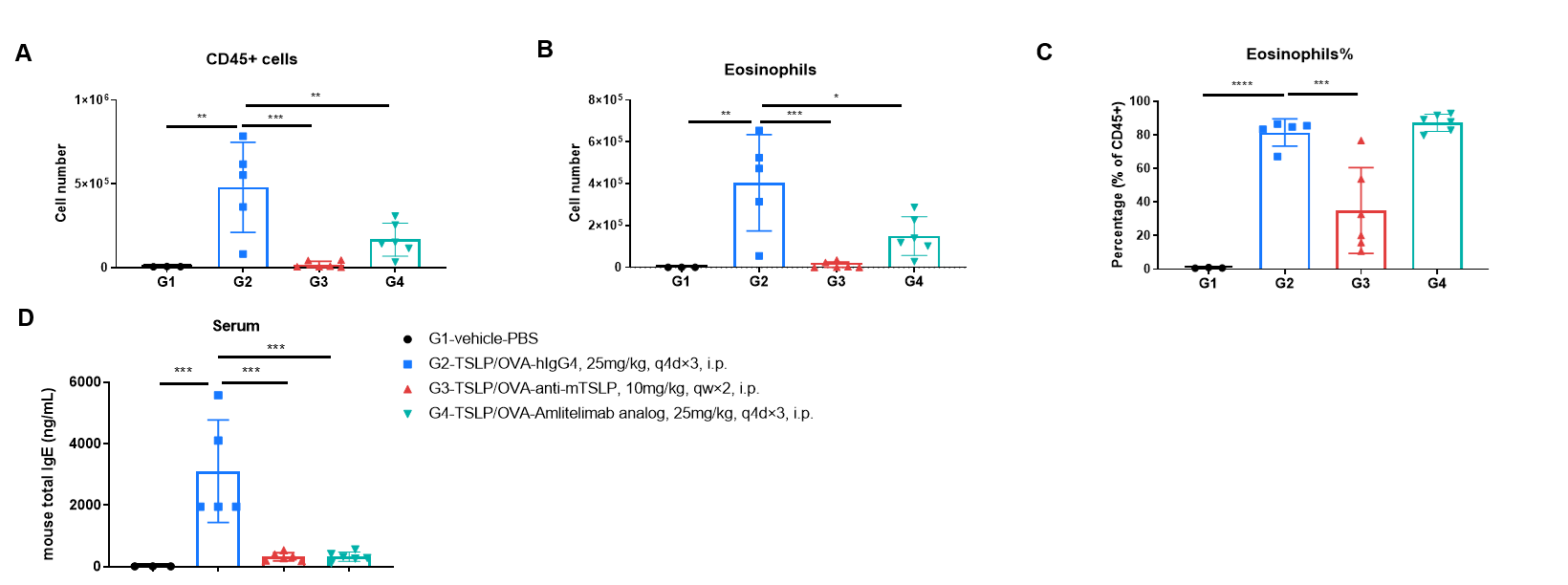
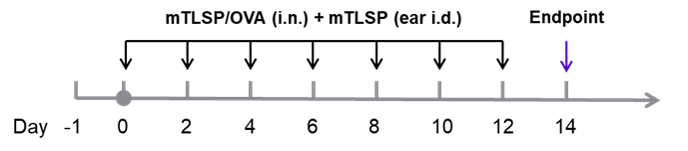
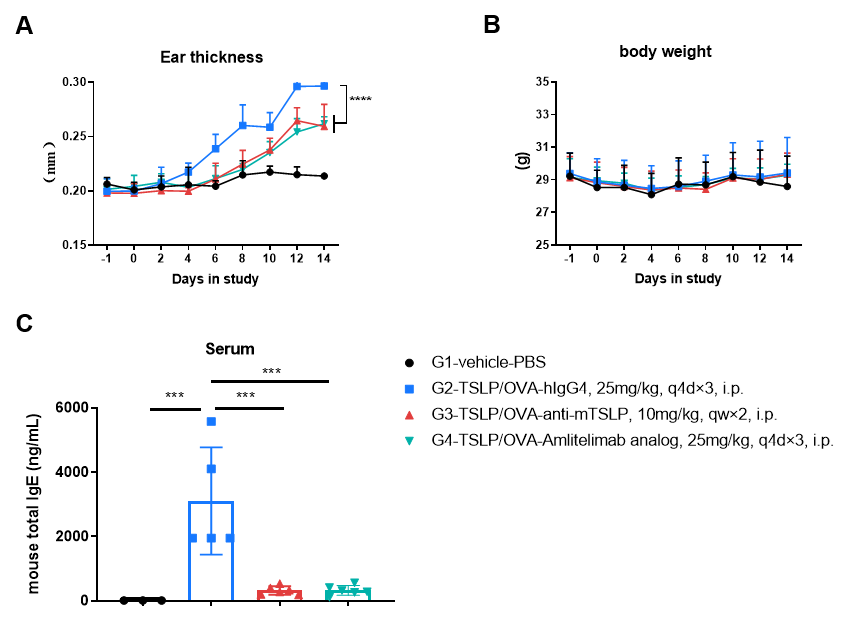
Analysis of immune cells in BALF and mouse total IgE in serum was performed in an mTSLP/OVA-induced asthma model. OX40/OX40L humanized mice (male, 11-week-old, n = 6) were immunized with mTSLP/OVA to induce asthma. Anti-human OX40L antibody (amlitelimab analog, synthesized in-house) was intraperitoneally injected from Day −1. (A & B) The numbers of CD45⁺ cells and eosinophils in BALF in the amlitelimab-treated group decreased significantly compared with the mTSLP/OVA-induced isotype-treated group. (D) Serum was collected at the study endpoint, and IgE levels were analyzed by ELISA. The results showed that total IgE levels in mice treated with amlitelimab (in-house) were significantly reduced compared with untreated mice. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
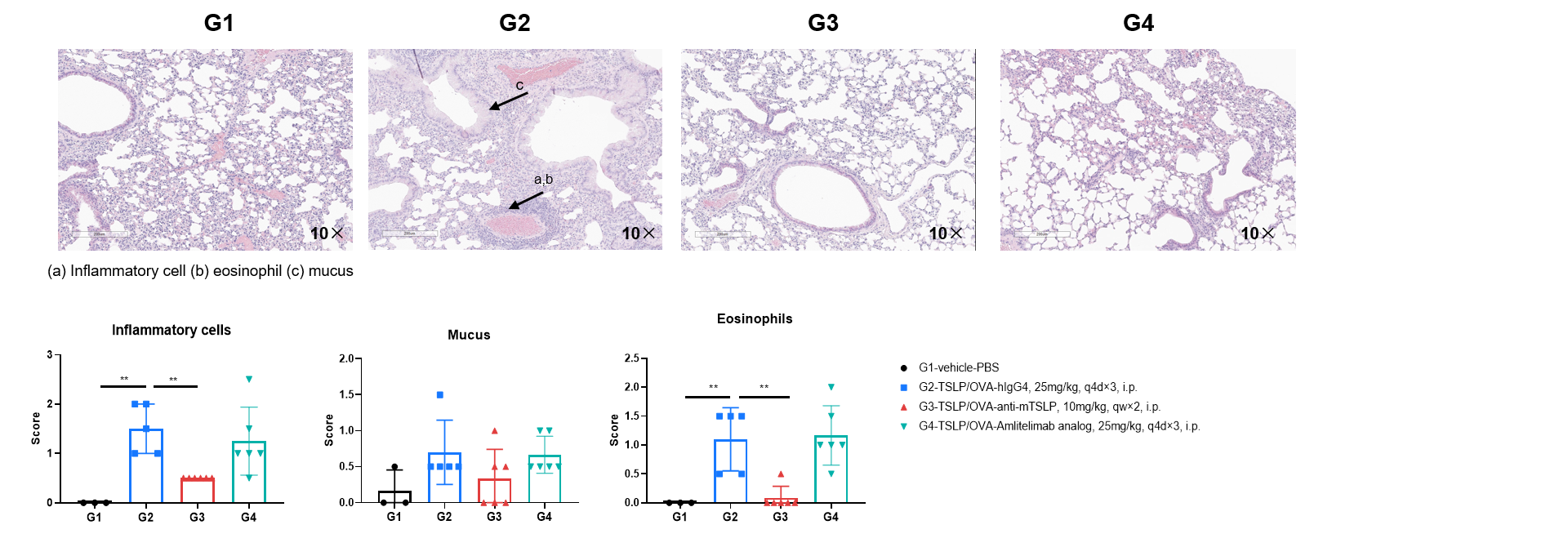
H&E staining of the asthma-like model in OX40/OX40L humanized mice. Lung tissues were collected at the study endpoint and analyzed with H&E staining. The results showed that inflammatory infiltration and mucus secretion in lung tissue were lower in mice treated with amlitelimab (in-house) than in untreated mice, indicating that OX40/OX40L humanized mice provide a powerful humanized OX40L preclinical model for in vivo evaluation of anti-human OX40L antibodies. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
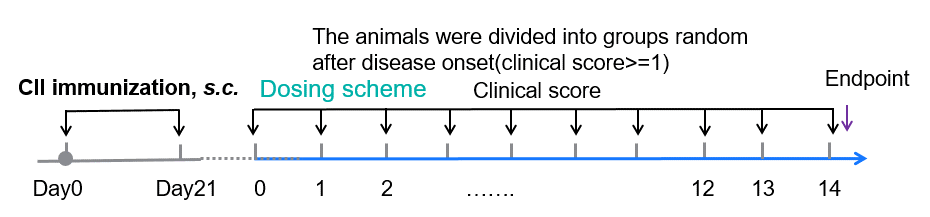
Experimental schedule for CIA induction and anti-human OX40L antibody treatment.
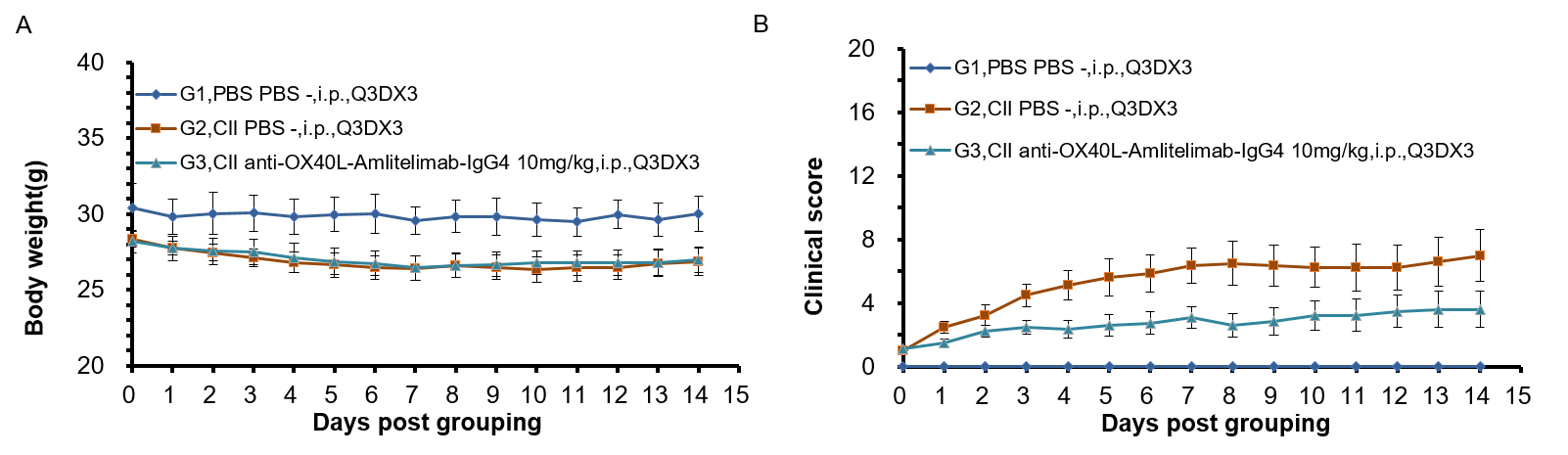
Experimental schedule for Induction of CIA and in vivo efficacy of anti-human OX40L antibody. 50 μL CⅡ emulsion injection subcutaneously at 2 points-the base of the tail and buttocks on day 0 and day 21 respectively. Animals with disease onset were grouped individually into G1-G3 between day 22 to day 30 while ensuring similar average clinical scores on the day of grouping. Mouse body weight (A) and clinical score (B) post grouping were shown for selected timepoints.
Q1: What are OX40/OX40L humanized mice used for?
They are used to evaluate anti-human OX40L antibodies and to study OX40–OX40L signaling in atopic dermatitis, asthma, and autoimmune disease.
Q2: Do OX40/OX40L humanized mice have normal immune-cell development?
Yes, leukocyte and T-cell subsets in spleen, blood, and lymph node are comparable to C57BL/6 mice.
Q3: Can these mice model atopic dermatitis and asthma?
Yes, they support the OXA-induced atopic dermatitis model and the OVA- or mTSLP/OVA-induced asthma models, and they respond well to anti-OX40L antibody treatment.
Q4: Do they express human or mouse OX40/OX40L?
Human OX40 and OX40L are expressed in the humanized mice, while mouse OX40/OX40L are detected only in wild-type controls.
Q5: Which drugs can be evaluated in this model?
Human OX40L-targeting antibodies such as amlitelimab and other OX40/OX40L pathway modulators.