
C57BL/6-Tfrctm1(TFRC)Bcgen Slc3a2tm1(SLC3A2)Bcgen/Bcgen • 113596
Background:
Verification:
Key Advantages
Applications
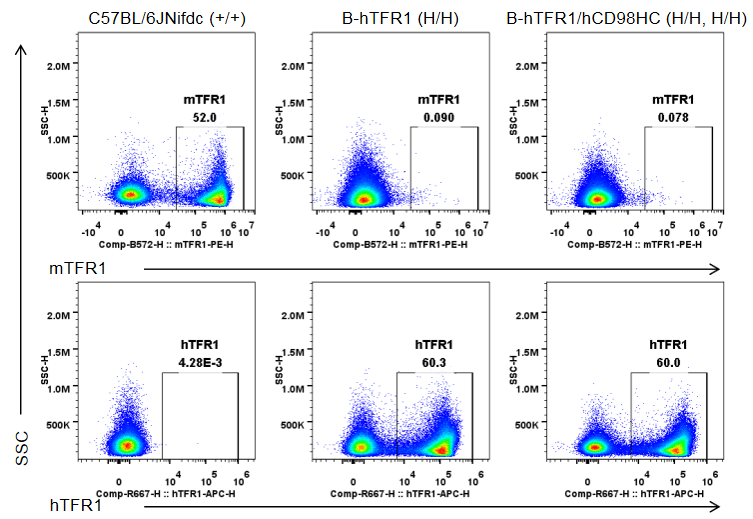
Strain-specific TFR1 expression was analyzed by flow cytometry in bone marrow erythrocytes from wild-type C57BL/6JNifdc mice (+/+), homozygous TFR1 humanized mice (H/H), and homozygous TFR1/CD98HC humanized mice (H/H, H/H). Bone marrow cells were analyzed with anti-mouse TFR1 antibody (BioLegend, 113808) and anti-human TFR1 antibody (BioLegend, 334108). Mouse TFR1 was detected only in erythrocytes of wild-type mice, whereas humanized TFR1 was exclusively detected in erythrocytes of homozygous TFR1 humanized mice and TFR1/CD98HC humanized mice, but not in wild-type mice.
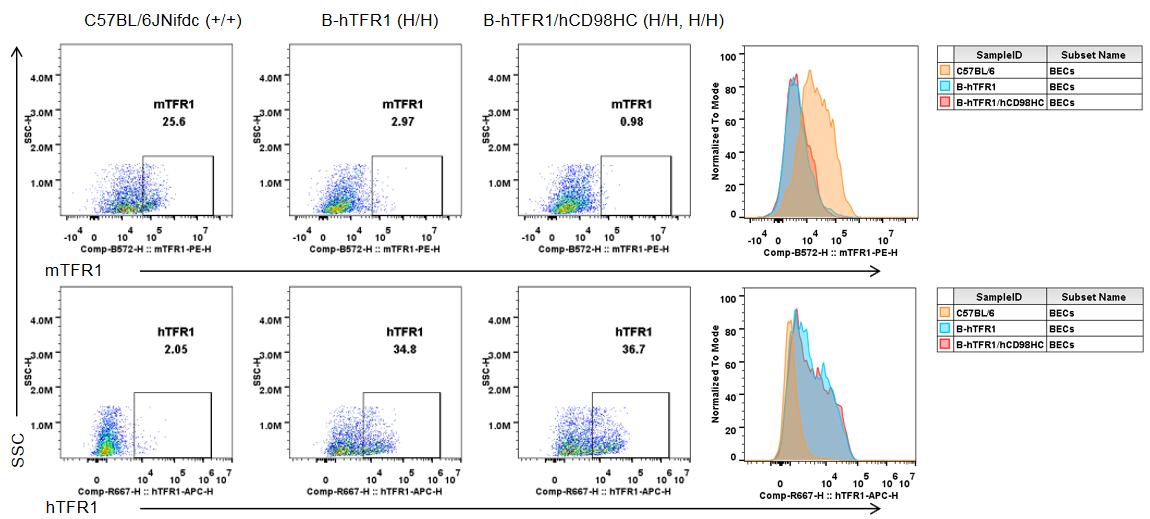
Brain cells were collected from wild-type C57BL/6JNifdc mice (+/+), homozygous TFR1 humanized mice (H/H), and homozygous TFR1/CD98HC humanized mice (H/H, H/H). Flow cytometry was performed using anti-mouse TFR1 antibody (BioLegend, 113808) and anti-human TFR1 antibody (BioLegend, 334108). Mouse TFR1 was detectable only in brain endothelial cells of wild-type mice, whereas humanized TFR1 was exclusively detected in brain endothelial cells of homozygous TFR1 humanized mice and TFR1/CD98HC humanized mice.
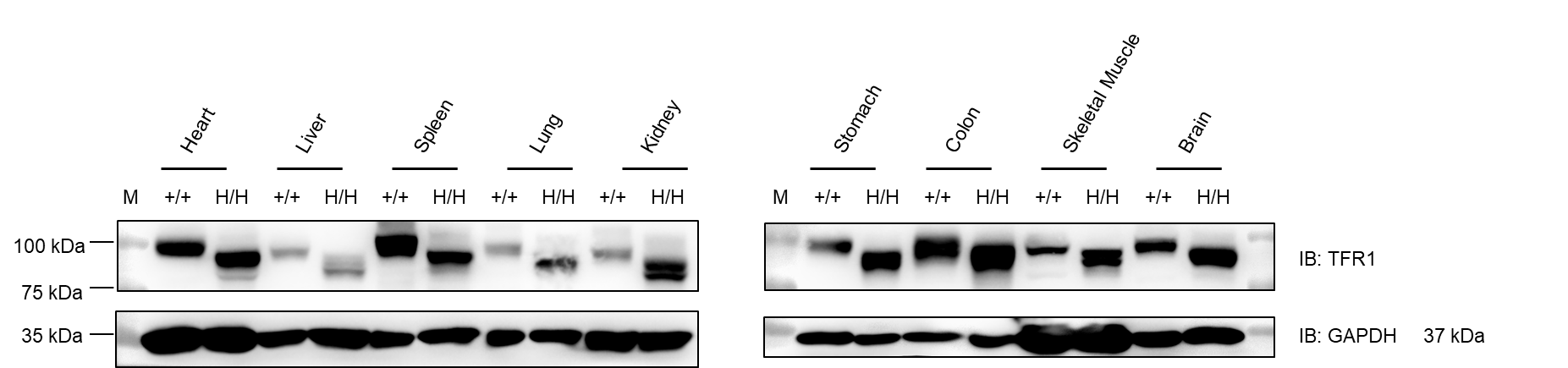
Western blot analysis was performed on tissue lysates from heart, liver, spleen, lung, kidney, stomach, colon, muscle, and brain of wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1 humanized mice (H/H). Samples were probed with anti-TFR1 antibody (Abcam, ab214039), which is cross-reactive between mouse and human TFR1. TFR1 protein was detectable in all examined tissues from both genotypes. GAPDH was used as a loading control. M: marker. Total protein loaded per lane: 40 μg.

Protein expression of TFR1 in the spinal cord, cerebral cortex, hippocampus, and cerebellum was analyzed by western blot in wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1/CD98HC humanized mice (H/H). Tissue lysates were probed with anti-transferrin receptor antibody (Abcam, ab214039), which is cross-reactive between species. TFR1 was detectable in CNS tissues from both wild-type and humanized mice. (A) Male. (B) Female. Total protein loaded per lane: 40 μg.
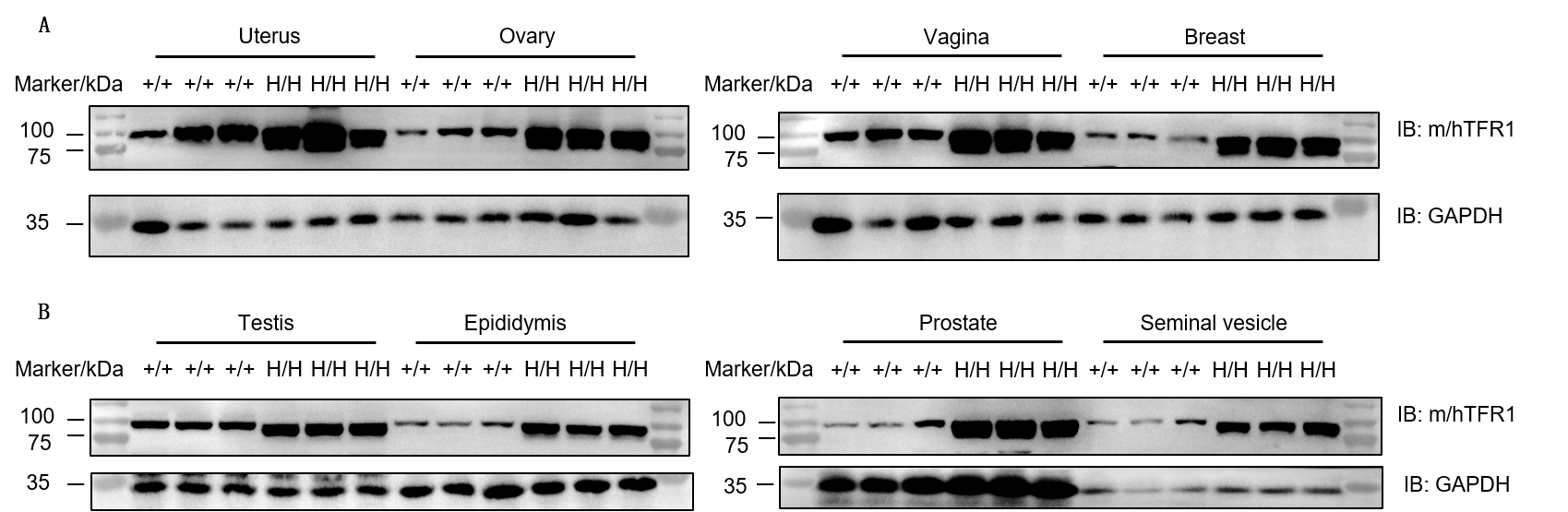
Western blot analysis of uterus, ovary, vagina, breast, testis, epididymis, prostate, and seminal vesicle was performed in wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1/CD98HC humanized mice (H/H) using anti-transferrin receptor antibody (Abcam, ab214039). TFR1 protein was detectable in all examined reproductive organs from both genotypes due to antibody cross-reactivity. Total protein loaded per lane: 30 μg.
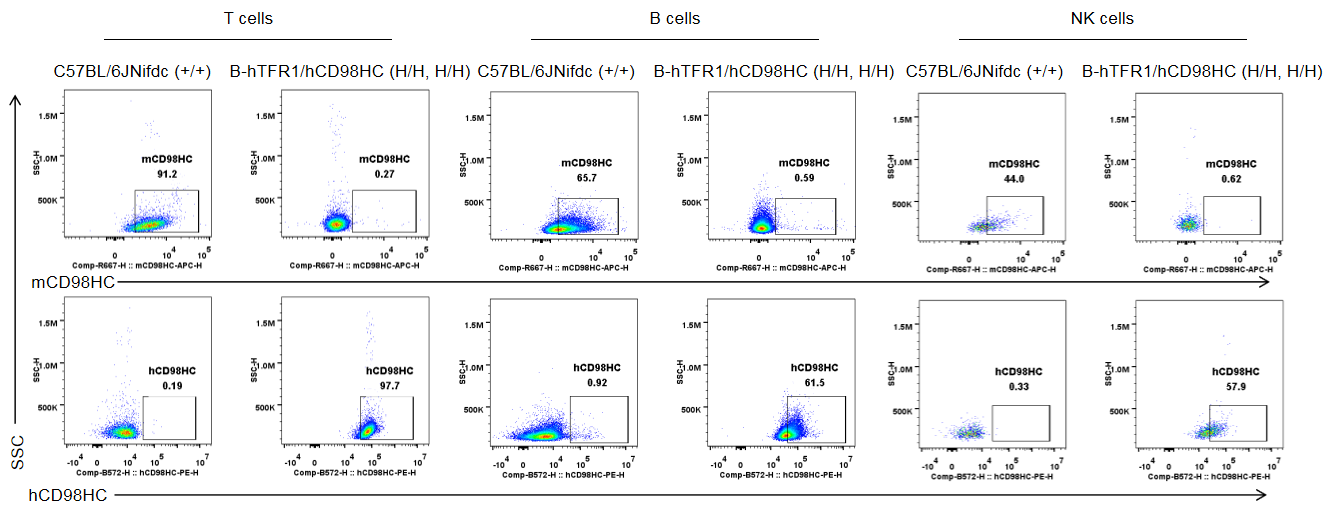
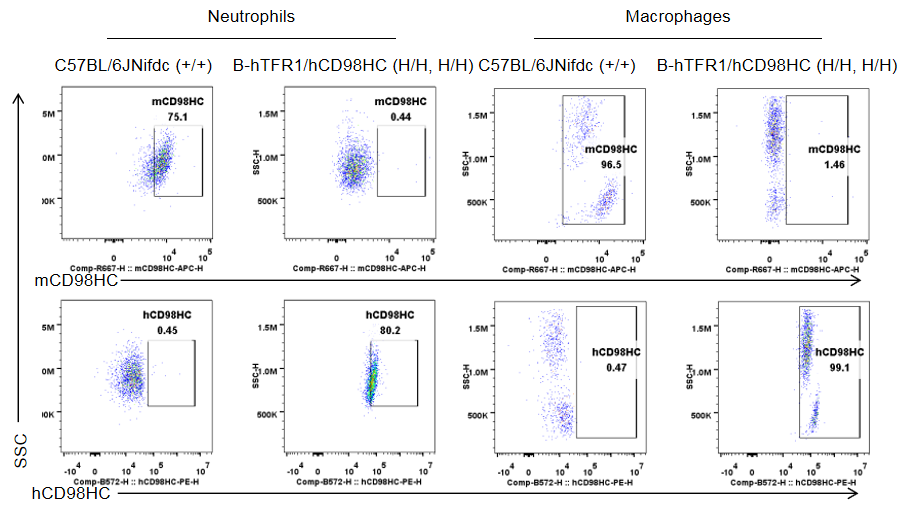
Blood cells were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1/CD98HC humanized mice (H/H, H/H) and analyzed by flow cytometry. Cells were stained with anti-mouse CD98HC antibody (BioLegend, 128211) and anti-human CD98HC antibody (231161-CD98BBBB-h1.L, produced in-house). Mouse CD98HC was detectable only in T cells, B cells, NK cells, neutrophils, and macrophages of wild-type mice, whereas humanized CD98HC was exclusively detected in corresponding leukocyte populations of homozygous TFR1/CD98HC humanized mice.
Strain specific CD98HC expression analysis in wild-type C57BL/6JNifdc and homozygous B-hTFR1/hCD98HC mice by flow cytometry. Blood cells were collected from wild-type C57BL/6JNifdc (+/+) and homozygous B-hTFR1/hCD98HC mice (H/H, H/H) and analyzed by flow cytometry with anti-mouse CD98HC antibody (Biolegend, 128211) and anti-human CD98HC antibody (231161-CD98BBBB-h1.L produced in-house). mCD98HC was only detectable in wild-type mice, and hCD98HC was exclusively detectable in homozygous mice but not in wild-type mice.
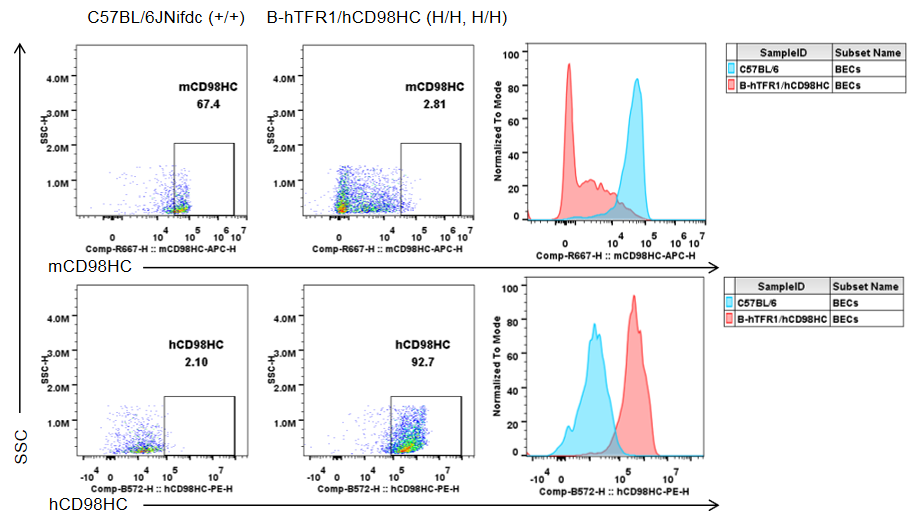
Brain cells were isolated from wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1/CD98HC humanized mice (H/H, H/H). Flow cytometry was performed using species-specific antibodies against mouse and human CD98HC. Mouse CD98HC was detected only in brain endothelial cells of wild-type mice, while human CD98HC was exclusively detected in brain endothelial cells of homozygous TFR1/CD98HC humanized mice.
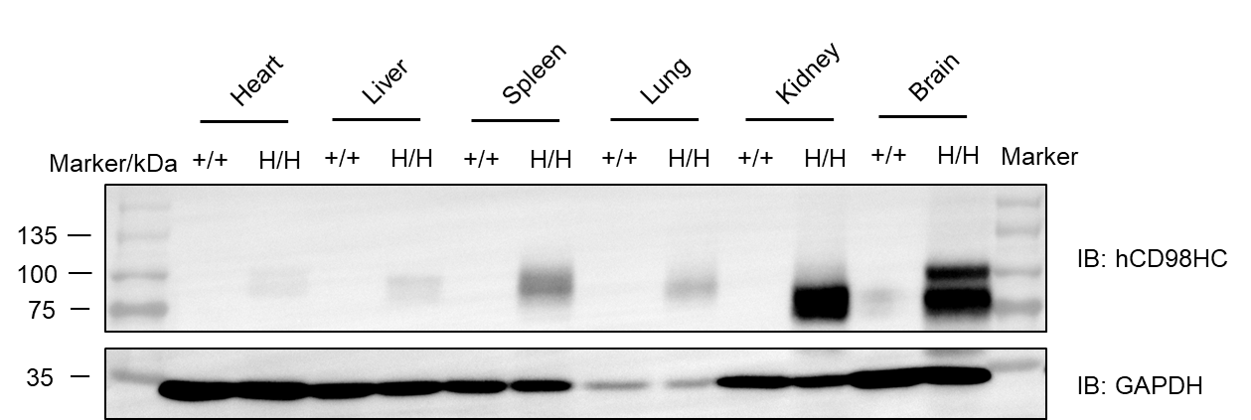
Western blot analysis was performed to evaluate human CD98HC protein expression in homozygous CD98HC humanized mice (H/H). Tissue lysates were collected from wild-type C57BL/6 mice and homozygous CD98HC humanized mice, and analyzed by western blot using a species-specific anti-human CD98 antibody (Abcam, ab307587). A total of 50 μg protein was loaded per lane. Human CD98HC protein was detectable in spleen, lung, kidney, and brain tissues from homozygous CD98HC humanized mice, but not in wild-type mice.
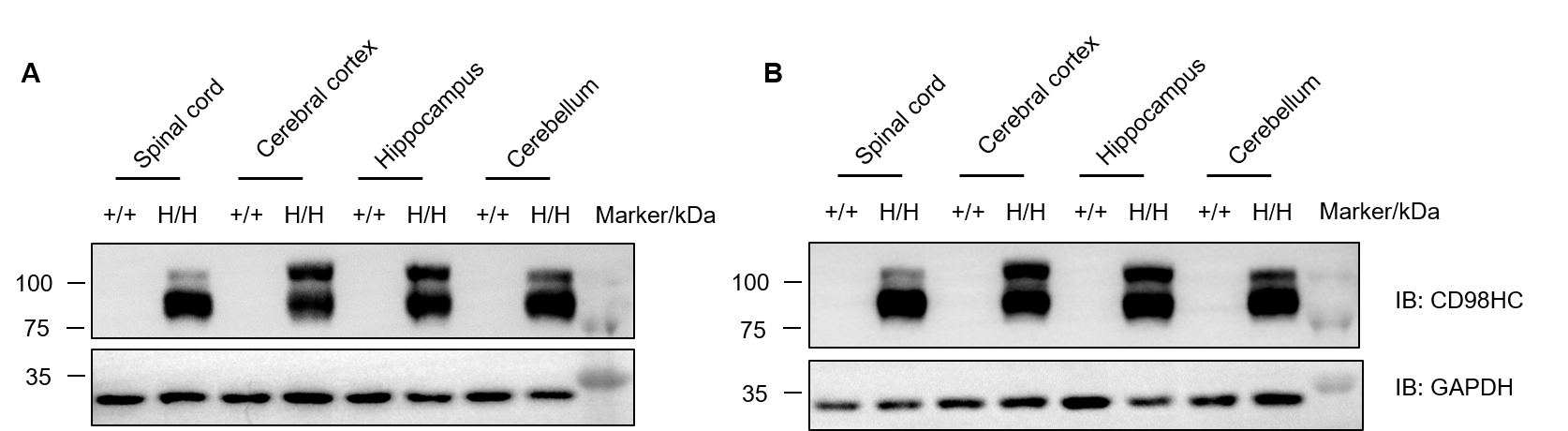
Protein expression of human CD98HC in the central nervous system was analyzed by western blot in wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1/CD98HC humanized mice (H/H). Tissue lysates from spinal cord, cerebral cortex, hippocampus, and cerebellum were collected and analyzed using anti-CD98 antibody (Abcam, ab307587). Human CD98HC protein was exclusively detected in all examined CNS regions of TFR1/CD98HC humanized mice, but not in wild-type controls. (A) Male mice. (B) Female mice. A total of 40 μg protein was loaded per lane.
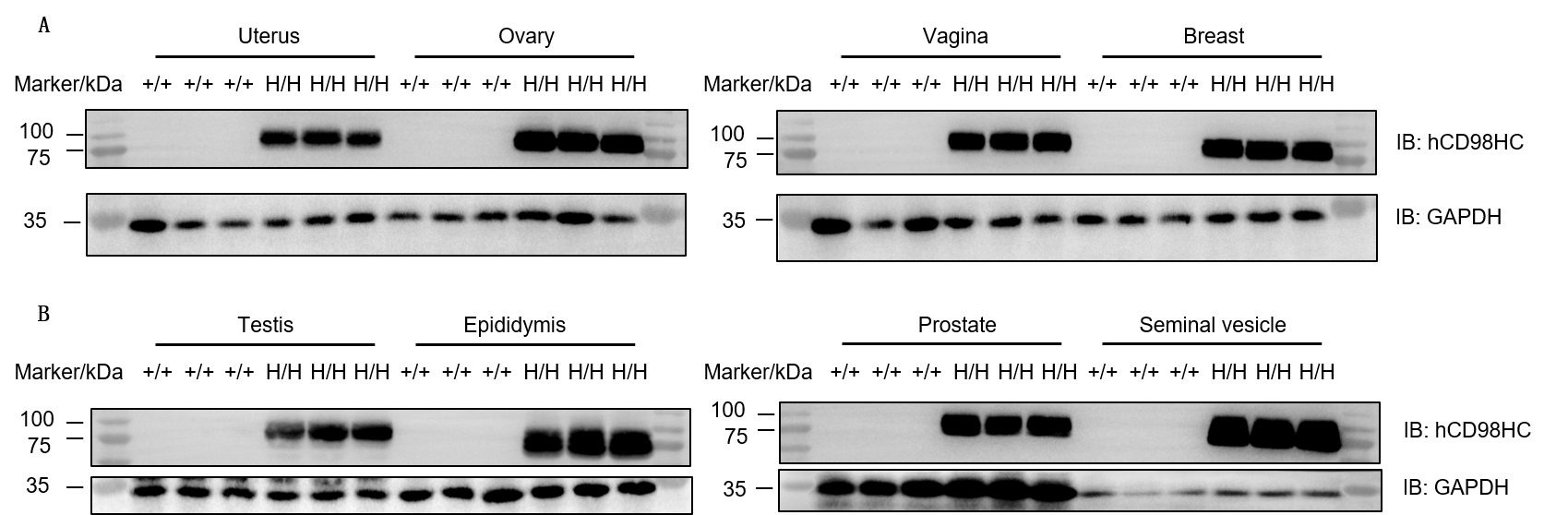
Protein expression of human CD98HC in reproductive organs was evaluated by western blot in wild-type C57BL/6JNifdc mice (+/+) and homozygous TFR1/CD98HC humanized mice (H/H). Tissue lysates were collected from uterus, ovary, vagina, breast, testis, epididymis, prostate, and seminal vesicle, and analyzed using anti-CD98 antibody (Abcam, ab307587). Human CD98HC protein was exclusively detected in all examined reproductive tissues of TFR1/CD98HC humanized mice, but not in wild-type mice. A total of 30 μg protein was loaded per lane.
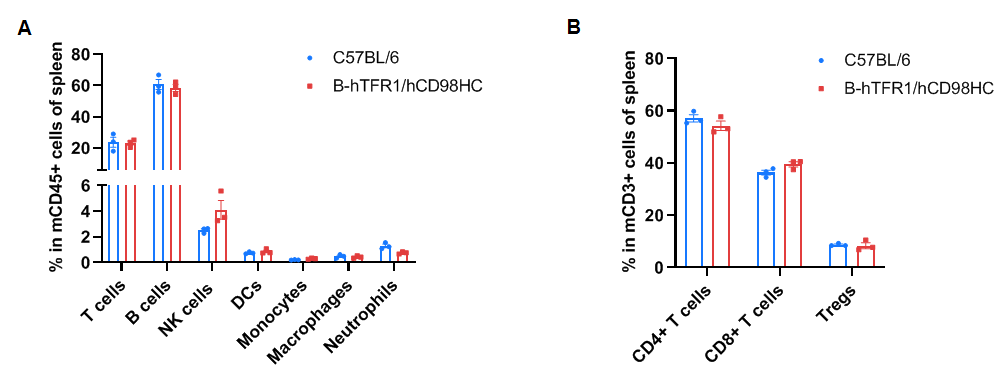
Splenocytes were isolated from wild-type C57BL/6JNifdc mice (female, n=3, 7-week-old) and homozygous TFR1/CD98HC humanized mice (female, n=3, 7-week-old). Flow cytometry analysis was performed to assess leukocyte subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes, macrophages, CD4⁺ T cells, CD8⁺ T cells, and Tregs were comparable between genotypes. Similar distributions were observed in blood and lymph nodes (data not shown). Values are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001). These results demonstrate that humanization of TFR1 and CD98HC does not alter immune cell development or distribution.
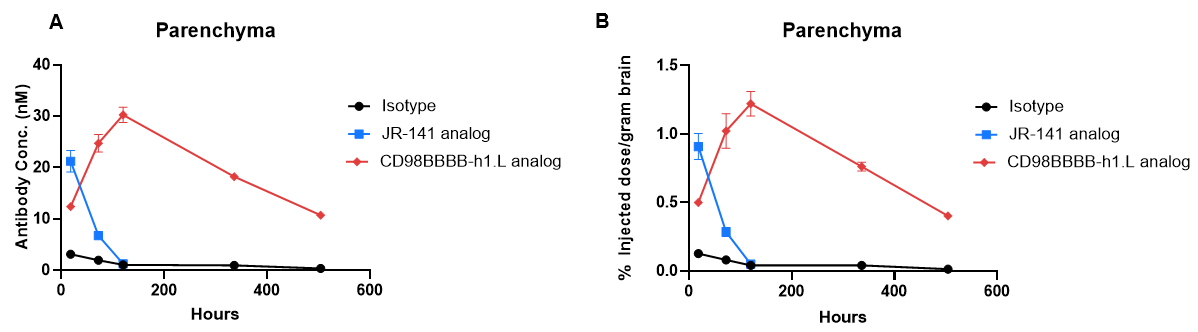
Homozygous TFR1/CD98HC humanized mice (female, 8-week-old, n=2) were intravenously injected with control IgG (10 mpk), anti-humanized TFR1 antibody (JR-141 analog, monovalent, produced in-house, 12.56 mpk), or anti-humanized CD98HC antibody (CD98BBBB-h1.L analog, monovalent, produced in-house, 13.3 mpk). Brain samples were collected for in vivo pharmacokinetic evaluation. Brain concentration and percentage of injected dose per gram of brain tissue were quantified. Anti-humanized TFR1 antibody showed higher brain exposure at 24 hours post-dose, whereas anti-humanized CD98HC antibody showed higher brain exposure at 72 hours post-dose. These results demonstrate that TFR1/CD98HC humanized mice enable uptake of intravenously administered antibodies targeting either TFR1 or CD98HC and support comparative evaluation of brain penetration efficiency for shuttle molecules. Data are shown as mean ± SEM.
Q1. What makes TFR1 / CD98HC humanized mice unique?
They co-express human TFR1 and CD98HC, enabling validation of BBB shuttle antibodies and biologics targeting TFR1 (TFRC/CD71) or CD98HC (SLC3A2).
Q2. Are these mice suitable for antibody validation in vivo?
Yes, the model is designed for direct testing of human-specific antibodies and biologics.
Q3. Does humanization affect immune system development?
No, immune cell composition and distribution remain comparable to wild-type mice.
Q4. What disease areas can be studied using this model?
BBB-crossing drugs for brain diseases, oncology, immunology, autoimmune diseases, and metabolic disorders.
Q5. Can this model be used for long-term studies?
Yes, normal growth and immune homeostasis support chronic dosing and longitudinal studies.