
C57BL/6N-Slc3a2tm1(SLC3A2)Bcgen/Bcgen • 110983
このページで
Key Advantages:
Model Validation:
Applications:
Gene targeting strategy for CD98HC humanized mice. The exons 2-10 of mouse CD98 gene that encode the extracellular domain were replaced by human CD98 exons 4-12 in CD98HC humanized mice.
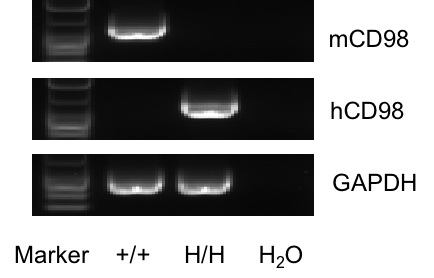
Strain-specific analysis of CD98 gene expression was conducted in wild-type (WT) and CD98HC humanized mice (B-hCD98HC) using RT-PCR. Mouse Cd98 mRNA was detectable only in the kidney of WT (+/+) mice, whereas human CD98 mRNA was exclusively detected in homozygous CD98HC humanized mice (H/H) but not in WT controls (+/+).
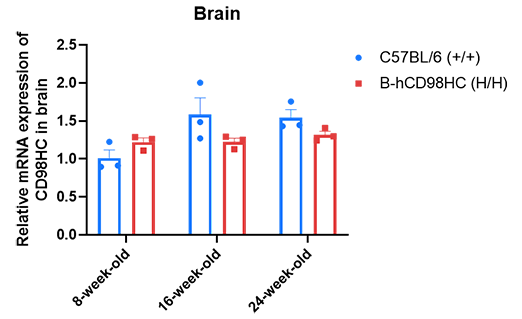
CD98HC mRNA expression was evaluated in the brains of wild-type C57BL/6 mice (+/+) (male, 8, 16, and 24 weeks old, n=3) and homozygous CD98HC humanized mice (H/H, male, same age groups, n=3). cDNA libraries were synthesized from brain RNA by reverse transcription, followed by PCR with CD98HC-specific primers. Expression levels of human CD98HC in homozygous mice were comparable to those in wild-type controls and remained consistent across different ages. Values are expressed as mean ± SEM.
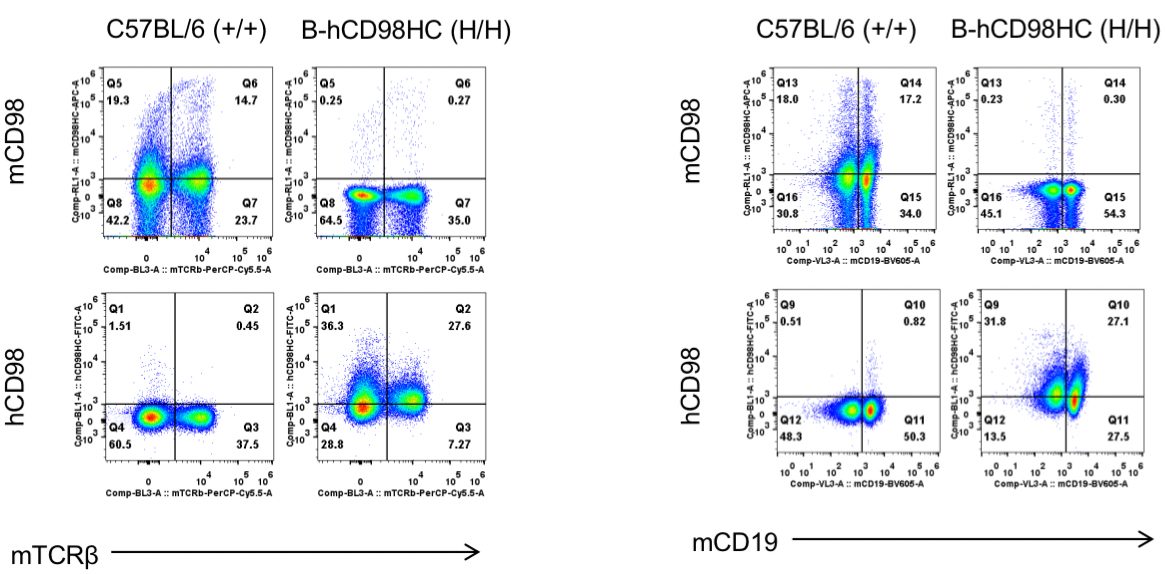
Strain-specific CD98HC protein expression was analyzed in wild-type (+/+) and homozygous CD98HC humanized mice (B-hCD98HC, H/H) by flow cytometry. Splenocytes were stained with a species-specific anti-CD98 antibody. Mouse CD98 was detectable in wild-type mice, while human CD98HC was exclusively expressed in homozygous CD98HC humanized mice
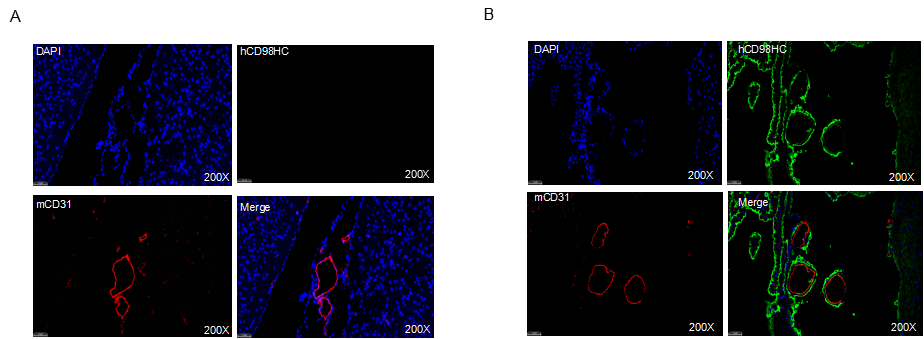
Strain-specific CD98HC expression was examined in brain tissues of wild-type and CD98HC humanized mice (B-hCD98HC, female, 8 weeks old) by immunofluorescence staining. Paraffin-embedded brain sections were stained with a species-specific anti-human CD98HC antibody (green) and co-stained with anti-mouse CD31 to visualize microvascular endothelial cells (red). Results showed human CD98HC expression exclusively on brain microvascular endothelium in homozygous CD98HC humanized mice, but not in wild-type controls.
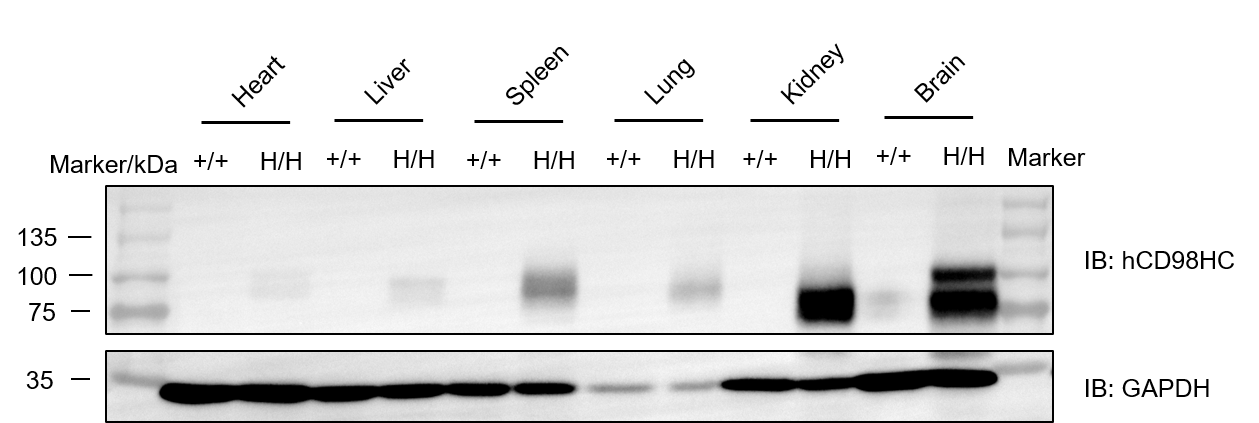
Western blot analysis was performed to detect human CD98HC protein expression in multiple tissues of homozygous CD98HC humanized mice (B-hCD98HC, H/H). Tissue lysates from spleen, lung, kidney, and brain were analyzed with a species-specific anti-human CD98 antibody (Abcam, ab307587), using 50 μg of total protein per lane. Human CD98HC protein was detectable in these tissues in homozygous mice, but not in wild-type controls.
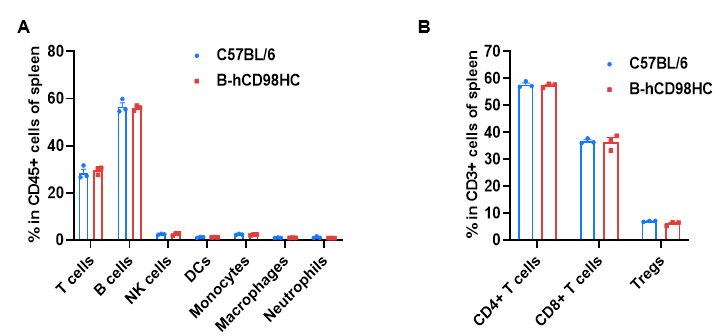
Splenocytes were isolated from wild-type C57BL/6 mice and homozygous CD98HC humanized mice (B-hCD98HC, female, 8 weeks old, n=3). Flow cytometry analysis was performed to evaluate leukocyte subpopulations.(A) Representative FACS plots. (B) Quantitative analysis of T cell subsets. Percentages of T cells, B cells, NK cells, dendritic cells, monocytes, macrophages, neutrophils, CD4⁺ T cells, CD8⁺ T cells, and Tregs were comparable between wild-type and CD98HC humanized mice, indicating that humanization of CD98HC does not alter immune cell distribution in the spleen. Values are expressed as mean ± SEM.
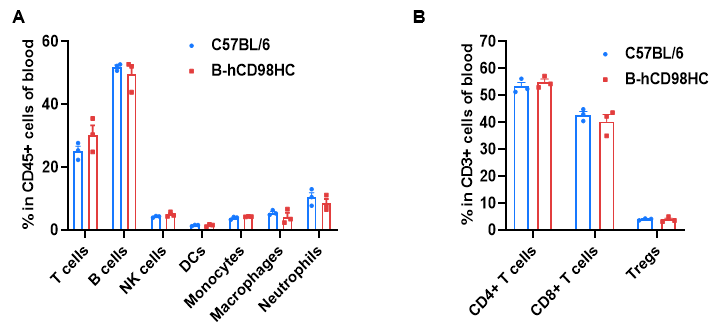
Blood cells were collected from wild-type C57BL/6 mice and homozygous CD98HC humanized mice (B-hCD98HC, female, 8 weeks old, n=3). Flow cytometry was performed to assess leukocyte subpopulations. (A) Representative FACS plots. (B) Quantitative analysis of T cell subsets. The percentages of T cells, B cells, NK cells, dendritic cells, monocytes, macrophages, neutrophils, CD4⁺ T cells, CD8⁺ T cells, and Tregs in homozygous CD98HC humanized mice were similar to wild-type controls, confirming that CD98HC humanization does not affect leukocyte composition in blood. Values are expressed as mean ± SEM.
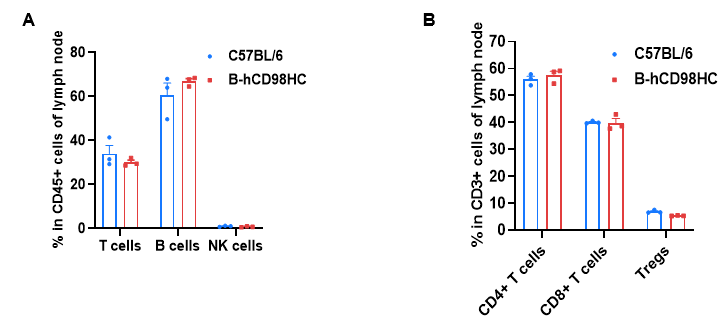
Leukocytes were isolated from lymph nodes of wild-type C57BL/6 mice and homozygous CD98HC humanized mice (B-hCD98HC, female, 8 weeks old, n=3). Flow cytometry analysis was performed to determine leukocyte subsets. (A) Representative FACS plots. (B) T cell subpopulation frequencies. Percentages of T cells, B cells, NK cells, CD4⁺ T cells, CD8⁺ T cells, and Tregs were consistent between CD98HC humanized mice and wild-type controls, indicating that CD98HC humanization does not alter immune cell development or distribution in lymph nodes. Values are expressed as mean ± SEM.
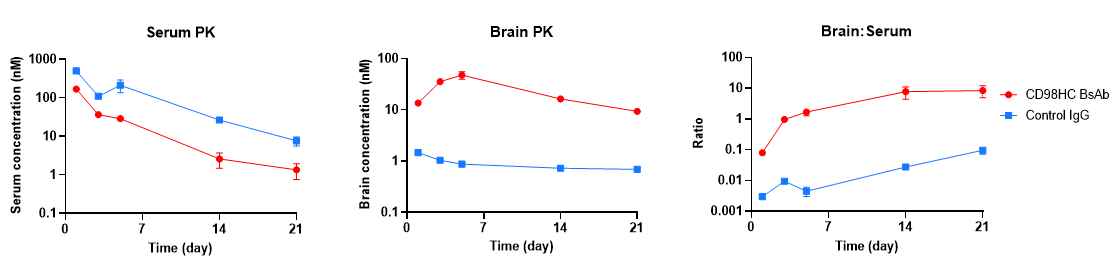
In vivo pharmacokinetic (PK) evaluation of anti-human CD98HC antibody was performed in CD98HC humanized mice (B-hCD98HC, female, 8 weeks old, n=2). Mice were injected via tail vein with either control IgG (10 mpk) or anti-human CD98HC antibody (CD98BBBB-h1.L analog, monovalent, produced in-house, 13.3 mpk). Brain and serum samples were collected for PK analysis. The anti-human CD98HC antibody showed higher serum clearance and enhanced brain exposure compared with control IgG. Brain-to-serum ratios confirmed active uptake of anti-human CD98HC antibody into the brain. Results demonstrate that CD98HC humanized mice provide a translational preclinical model for evaluating CNS-targeted therapeutic antibody delivery. Graphs represent mean ± SEM.
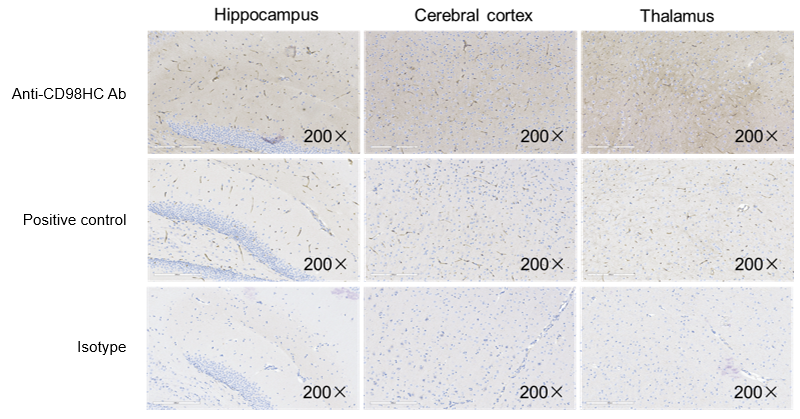
Immunohistochemistry (IHC) analysis was performed to evaluate antibody penetration. CD98HC humanized mice (B-hCD98HC) were injected via tail vein with control IgG (10 mpk) or anti-human CD98HC antibody (CD98BBBB-h1.L analog, monovalent, produced in-house, 13.3 mpk). Brain compartments were collected for IHC staining 120 hours post-injection. Results confirmed that the anti-human CD98HC antibody penetrates the brain parenchyma of CD98HC humanized mice, validating this model for CNS drug delivery studies.
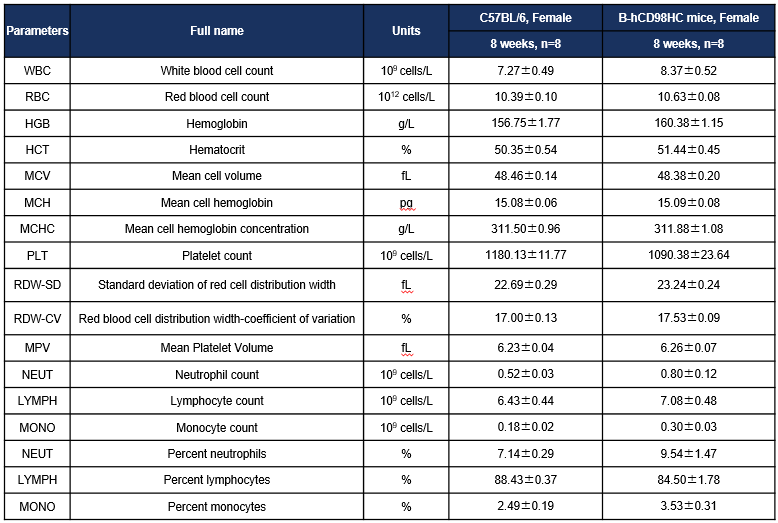
Complete blood count (CBC) results of CD98HC humanized mice. Values = mean ± SD.
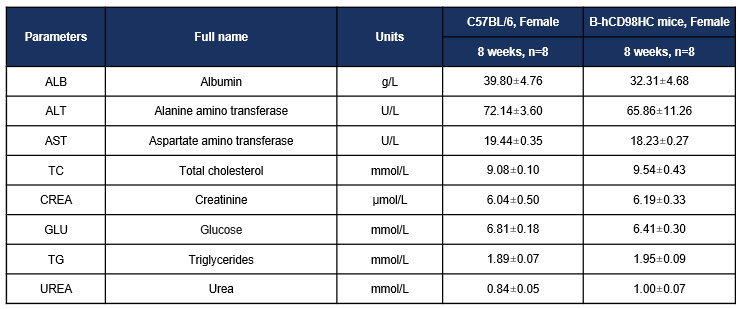
Biochemistry test results of CD98HC humanized mice confirm normal serum biochemical markers. Values = mean ± SD.
Q1: What are CD98HC humanized mice?
CD98HC humanized mice (B-hCD98HC) are engineered to replace murine Cd98 with human CD98 (SLC3A2), enabling expression of human CD98 heavy chain (CD98HC) in immune and CNS tissues.
Q2: Why are CD98HC humanized mice important?
CD98HC regulates integrin signaling and amino acid transport, and is highly expressed in proliferating cells. This model allows translational research in oncology, immunology, and CNS drug delivery.
Q3: Can CD98HC humanized mice be used for antibody validation?
Yes. Anti-human CD98HC antibodies can be tested for binding, pharmacokinetics, and brain penetration in vivo, making this model highly relevant for preclinical drug evaluation.
Q4: Do CD98HC humanized mice show normal immune function?
Yes. Flow cytometry confirmed that the distribution of leukocyte subsets in spleen, blood, and lymph nodes is comparable to wild-type controls, with no alterations in immune homeostasis.
Q5: What diseases or conditions can be studied using CD98HC humanized mice?
This model is particularly valuable for cancer, autoimmune diseases, and neurological disorders, and for testing the ability of therapeutic antibodies to cross the blood-brain barrier.