
C57BL/6JNifdc-Fgfr3tm1(FGFR3*P301S)Bcgen/Bcgen • 113287
Gene targeting strategy for B-hFGFR3*G380R mice. The exons 2-18 of mouse Fgfr3 gene that encode the whole molecule (ATG to STOP codon), including 3’UTR were replaced by human counterparts in B-hFGFR3*G380R mice. The promoter and 5’UTR region of the mouse gene are retained. The human FGFR3 expression is driven by endogenous mouse Fgfr3 promoter, while mouse Fgfr3 gene transcription and translation will be disrupted.
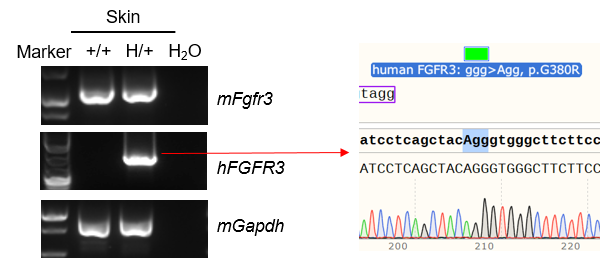
Strain specific analysis of FGFR3 mRNA expression in wild-type C57BL/6 mice and B-hFGFR3*G380R mice by RT-PCR. Skin RNA were isolated from wild-type C57BL/6 mice (+/+) and heterozygous B-hFGFR3*G380R mice (H/+), then cDNA libraries were synthesized by reverse transcription, followed by PCR with mouse or human FGFR3 primers. Mouse Fgfr3 mRNA were both detectable in wild-type C57BL/6 mice and heterozygous B-hFGFR3*G380R mice. Human FGFR3 mRNA was detectable only in heterozygous B-hFGFR3*G380R mice but not in wild-type mice.
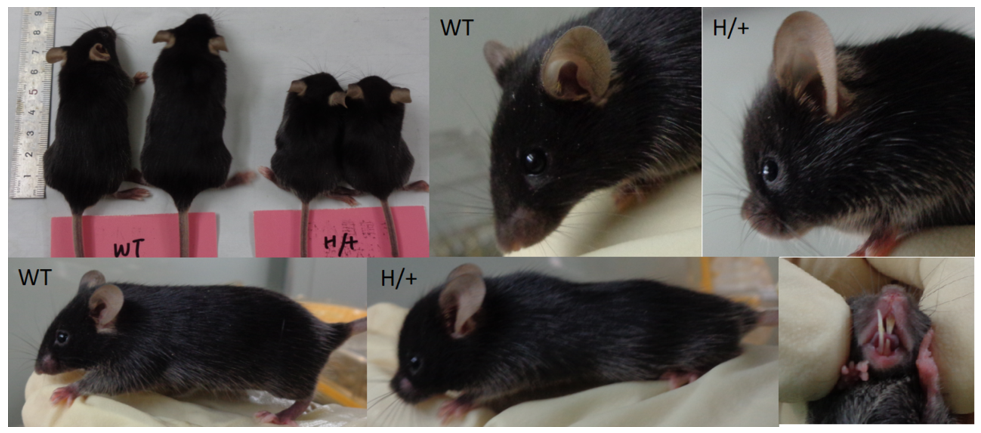
Phenotypic analysis of heterozygous B-hFGFR3*G380R mice: Heterozygous B-hFGFR3*G380R mice (6-week-old) exhibit a short body, rounded head, short snout, curved spine and protruding incisors.
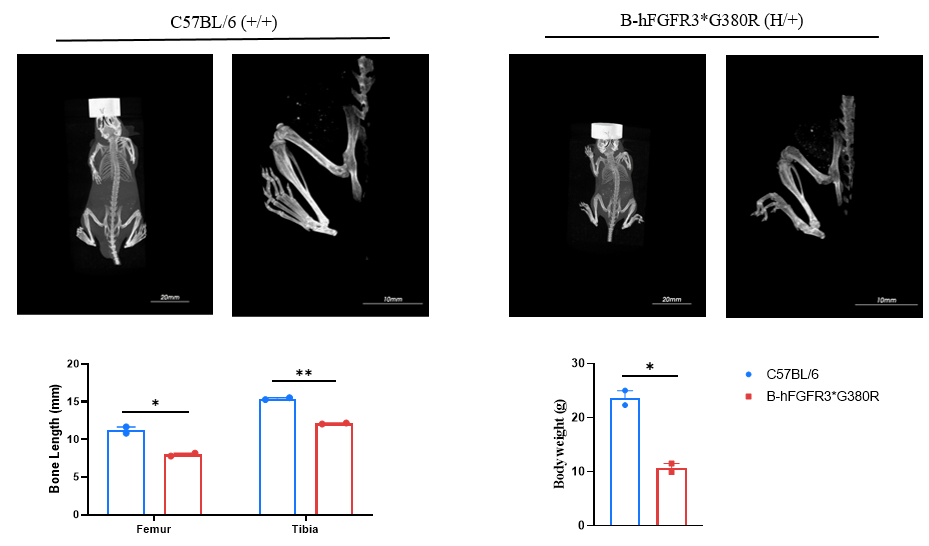
Micro-CT analysis of heterozygous B-hFGFR3*G380R mice: The length of the femurs and tibiae in heterozygous B-hFGFR3*G380R mice (6-week-old) are significantly shorter than those in the wild-type C57BL/6JNifdc mice (6-week-old).

In Vivo Efficacy of Vosoritide-analog in B-hFGFR3*G380R Mice. (A) Schematic illustration of the experimental schedule. Heterozygous B-hFGFR3*G380R mice (male, n=6) in each group were subcutaneously injected with vosoritide-analog (0.8 mg/kg, MCE, HY-P3503) for 28 days, starting from postnatal day 7. On postnatal days 22 and 35, micro-CT analysis was conducted to assess femur and tibia length as well as whole-body length. (B) Temporal progression of body weight from postnatal day 7 to day 34. (C) Femur length, tibia length, and whole-body length on postnatal days 22 and 35. Vosoritide-analog promotes the growth and development of axial and limb bones, and exerts a positive impact on ameliorating the disease in B-hFGFR3*G380R mice models of achondroplasia. Values are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
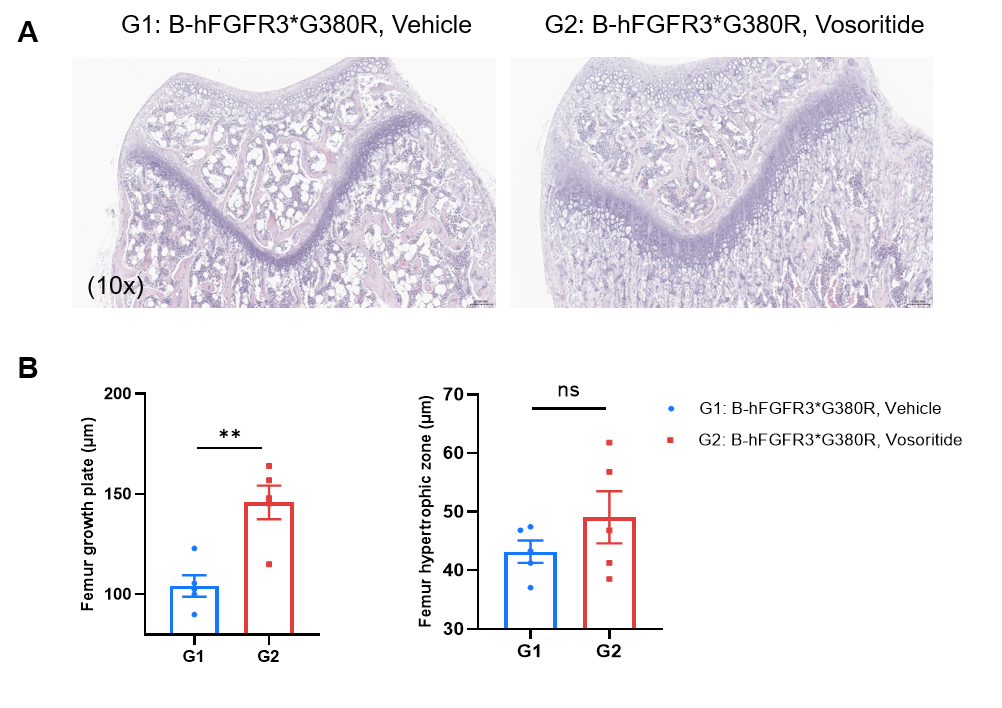
In Vivo Efficacy of Vosoritide-analog in B-hFGFR3*G380R Mice. (A) Representative H&E-stained sections of the distal femur growth plates (10× magnification) in B-hFGFR3*G380R mice treated with or without Vosoritide-analog. (B) Quantification of femur growth plate width (top) and femur hypertrophic zone length (bottom). Vosoritide-analog increases the width of the femoral growth plate. Data are presented as mean ± SD. Values are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA. P < 0.05, P < 0.01, *P < 0.001.
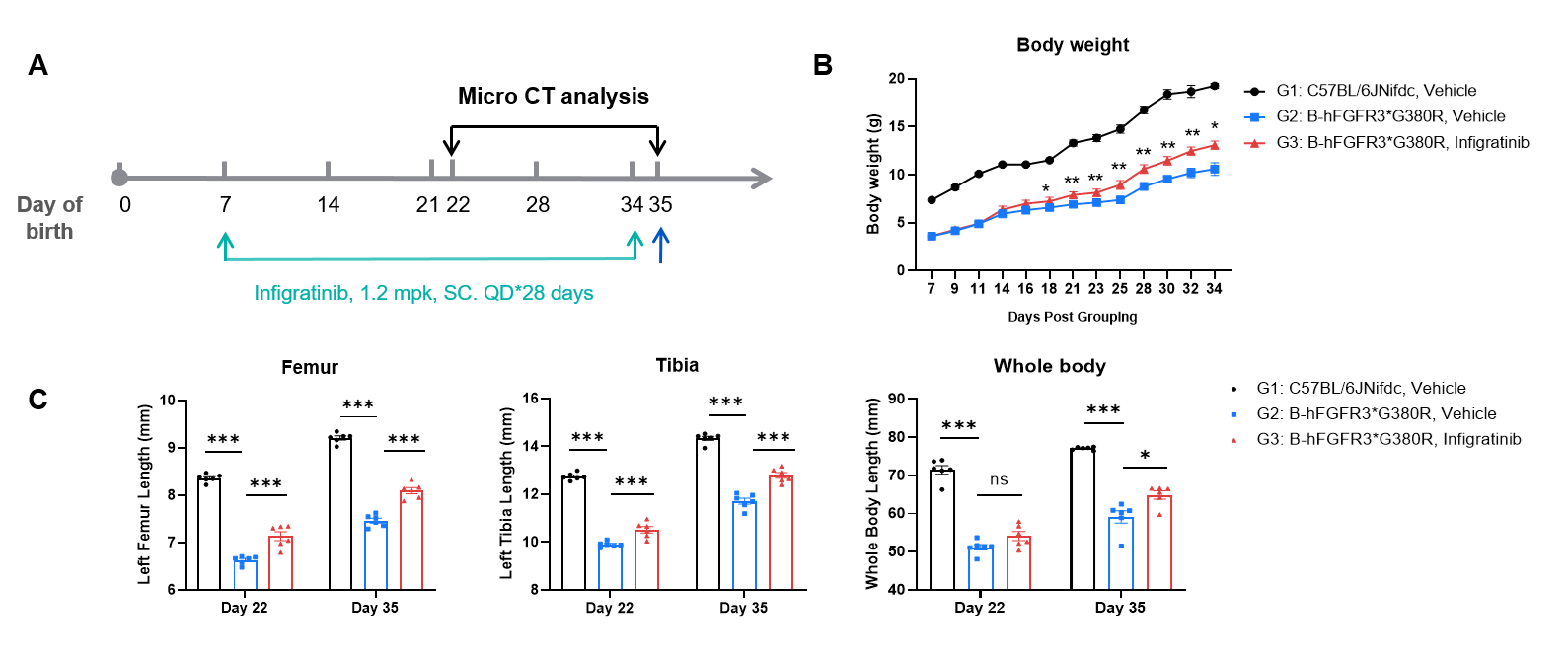
In Vivo Efficacy of Infigratinib-analog in B-hFGFR3*G380R Mice. (A) Schematic illustration of the experimental schedule. Wild-type C57BL/6JNifdc mice (male, n=6) and heterozygous B-hFGFR3*G380R mice (male, n=6) in each group were subcutaneously injected with Infigratinib-analog (1.2 mg/kg, provided by a client) for 28 days, starting from postnatal day 7. On postnatal days 22 and 35, micro-CT analysis was conducted to assess femur and tibia length as well as whole-body length. (B) Temporal progression of body weight from postnatal day 7 to day 34. (C) Femur length, tibia length, and whole-body length on postnatal days 22 and 35. Infigratinib-analog promotes the growth and development of axial and limb bones, and exerts a positive impact on ameliorating the disease in B-hFGFR3*G380R mice models of achondroplasia. Values are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
This data was generated through collaborative validation with a client.

In Vivo Efficacy of Infigratinib-analog in B-hFGFR3*G380R Mice. (A) Representative H&E-stained sections of the distal femur growth plates (10× magnification) in wild-type C57BL/6JNifdc mice and B-hFGFR3*G380R mice treated with infigratinib-analog. (B) Quantification of femur growth plate width (top) and femur hypertrophic zone length (bottom). Infigratinib-analog increases the width of the femoral growth plate. Data are presented as mean ± SD. Values are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA. P < 0.05, P < 0.01, *P < 0.001.
This data was generated through collaborative validation with our client.