
C57BL/6-Pdcd1tm1(PDCD1)Bcgen Cd274tm1(CD274)Bcgen/Bcgen • 120522

| Product name | B-hPD-1/hPD-L1 mice |
|---|---|
| Catalog number | 120522 |
| Strain name | C57BL/6-Pdcd1tm1(PDCD1)Bcgen Cd274tm1(CD274)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 5133,29126 (Human) |
| Aliases | PD1; PD-1; CD279; SLEB2; hPD-1; hPD-l; hSLE1; ADMIO4; AIMTBS; B7-H; B7H1; PDL1; PD-L1; ADMIO5; hPD-L1; PDCD1L1; PDCD1LG1 |
PD-1 (programmed cell death protein 1, PDCD1) and its ligand PD-L1 (CD274, also known as B7-H1) are pivotal immune checkpoint molecules that regulate T cell activation, tolerance, and peripheral immune homeostasis. While PD-1 is primarily expressed on activated T cells, B cells, and myeloid cells, PD-L1 is broadly expressed not only on antigen-presenting cells (APCs) but also on non-hematopoietic tissues and many tumor cells.
Through PD-1/PD-L1 interactions, tumors exploit this pathway to suppress T cell function, leading to immune evasion. Overexpression of PD-L1 is strongly associated with poor prognosis in cancers such as melanoma, lung cancer, and renal cell carcinoma, making PD-L1 a critical biomarker and therapeutic target in oncology. The success of anti-PD-L1 antibodies (e.g., atezolizumab, durvalumab, avelumab) highlights its importance in clinical immunotherapy.
In PD-1/PD-L1 humanized mice, the murine Pdcd1 and Cd274 genes are replaced with the corresponding human PDCD1 and CD274 sequences, enabling the expression of human PD-1 and PD-L1 under physiological promoters. This design maintains normal immune cell development and immune homeostasis while allowing for specific evaluation of human PD-1/PD-L1 interactions in vivo.
This PD-1/PD-L1 humanized mouse model provides a robust and translationally relevant platform for preclinical efficacy testing, antibody validation, biomarker discovery, and combination therapy development targeting the PD-1/PD-L1 axis.
Key Advantages:
Validation:
Applications:
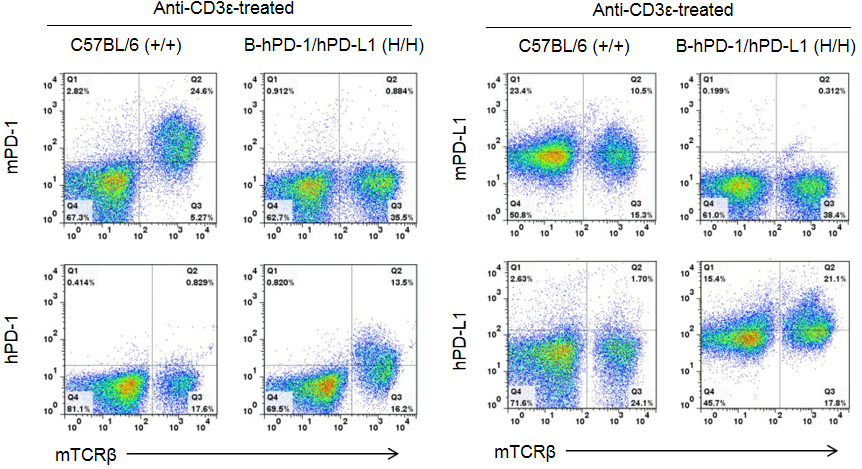
Strain-specific analysis of PD-1 and PD-L1 expression in homozygous PD-1/PD-L1 humanized mice by flow cytometry. Splenocytes were collected from WT and homozygous PD-1/PD-L1 (H/H) humanized mice stimulated with anti-CD3ε in vivo. Cells were analyzed by flow cytometry using species-specific anti-PD-1 and anti-PD-L1 antibodies. Mouse PD-1 and PD-L1 were exclusively detected in WT mice, whereas human PD-1 and PD-L1 were exclusively detected in homozygous PD-1/PD-L1 humanized mice, but not in WT controls. Flow cytometry confirmed human-specific expression of PD-1 and PD-L1 in PD-1/PD-L1 humanized mice, validating this model for checkpoint inhibitor research.
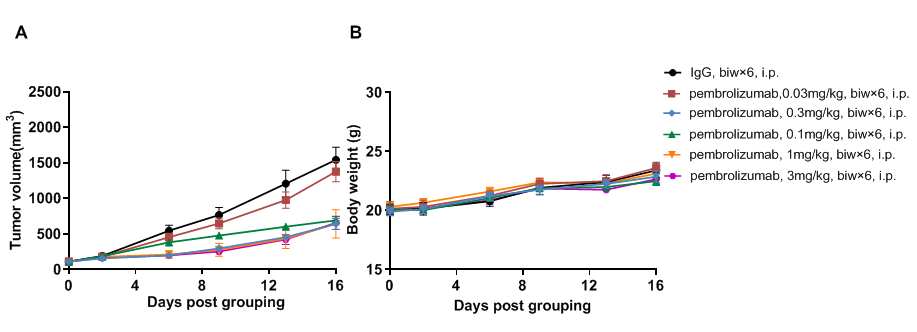
Antitumor activity of anti-human PD-1 antibody in PD-1/PD-L1 humanized mice. (A) The anti-human PD-1 antibody pembrolizumab (in-house) significantly inhibited tumor growth of B-hPD-L1 MC38 plus cells in PD-1/PD-L1 humanized mice. Murine colon cancer B-hPD-L1 MC38 plus cells (5 × 10^5) were subcutaneously implanted into homozygous PD-1/hPD-L1 humanized mice (male, 8 weeks old, n=5). Mice were randomized when tumors reached approximately 80 mm³ and treated with pembrolizumab at indicated doses and schedules. (B) Body weight was monitored during treatment. Different doses of pembrolizumab inhibited tumor growth to varying degrees, confirming that PD-1/PD-L1 humanized mice provide a robust in vivo model for evaluation of anti-human PD-1 antibodies. PD-1/PD-L1 humanized mice demonstrated dose-dependent tumor inhibition by anti-hPD-1 antibody, supporting their use in preclinical PD-1 drug development. Data are expressed as mean ± SEM.
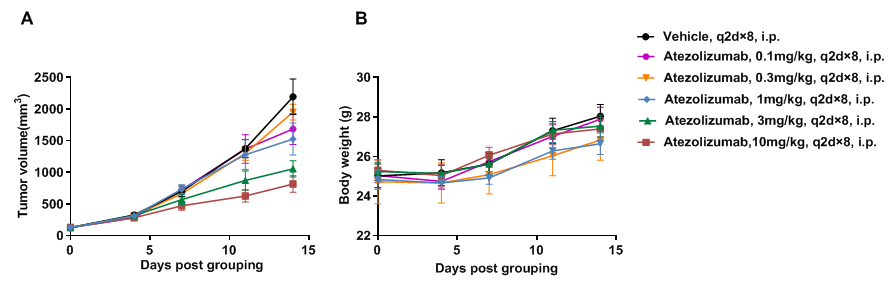
Antitumor activity of anti-human PD-L1 antibody in PD-1/PD-L1 humanized mice. (A) The anti-human PD-L1 antibody atezolizumab (in-house) inhibited tumor growth in PD-1/PD-L1 humanized mice bearing B-hPD-L1 MC38 plus tumors. Tumor cells (5 × 10^5) were subcutaneously implanted into homozygous PD-1/PD-L1 humanized mice (male, 5 weeks old, n=5). Mice were randomized at ~100 mm³ tumor volume and treated with atezolizumab at indicated doses and schedules. (B) Body weight was recorded during treatment. Tumor growth inhibition was observed at different dose levels, demonstrating that PD-1/PD-L1 humanized mice are a reliable preclinical model for testing anti-human PD-L1 antibodies. The model supports evaluation of anti-hPD-L1 therapeutics, with atezolizumab treatment leading to tumor growth suppression. Data are expressed as mean ± SEM.
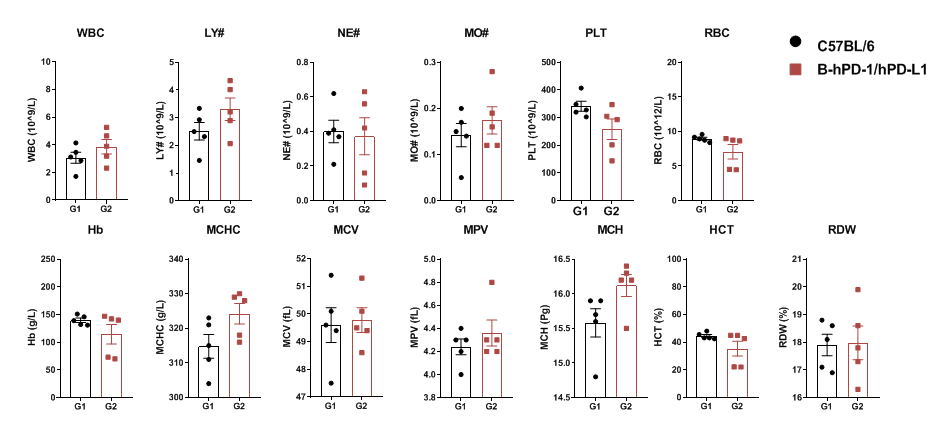
Complete blood counts (CBC) were performed on blood collected from female C57BL/6 and PD-1/PD-L1 humanized mice (n=5, 6 weeks old). No significant differences were observed across major hematological parameters between the two groups.
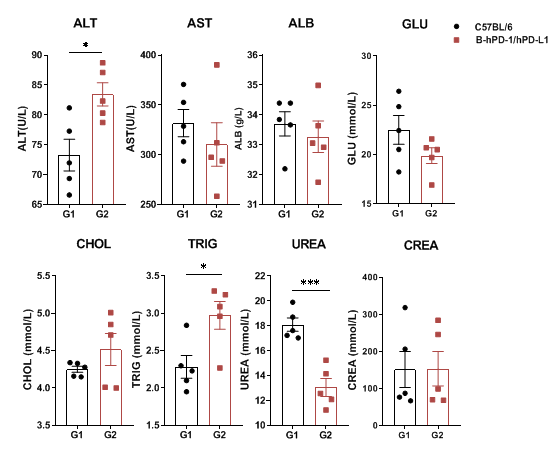
Serum was collected from C57BL/6 and PD-1/PD-L1 humanized mice (n=3, 6 weeks old) for biochemical analysis. No significant differences were detected in AST, ALB, GLU, CHOL, or CREA between groups. However, ALT, TRIG, and UREA levels showed significant changes compared to WT mice. PD-1/PD-L1 humanized mice show largely normal blood chemistry, with only minor differences compared to WT controls. Values are expressed as mean ± SEM.
Serum from the C57BL/6 and PD-1/PD-L1 humanized mice (n = 3, 6 week-old) was collected and analyzed for levels of biochemistry. No significant differences were detected on AST, ALB, GLU, CHOL, CREA measurement between C57BL/6 and PD-1/PD-L1 humanized mice. Values are expressed as mean ± SEM.
Q1: What are PD-1/PD-L1 humanized mice used for?
A1: They are used for preclinical testing of checkpoint inhibitors targeting PD-1 and PD-L1, including monotherapy and combination therapy strategies.
Q2: Why use a double humanized model instead of single humanized PD-1 or PD-L1 mice?
A2: The double humanized model better mimics the tumor immune microenvironment, allowing evaluation of therapeutic interactions between PD-1 and PD-L1.
Q3: Do PD-1/PD-L1 humanized mice respond to clinical checkpoint inhibitors?
A3: Yes, they are validated for antibodies including nivolumab, pembrolizumab, and atezolizumab.
Q4: Can this model be combined with tumor xenograft studies?
A4: Yes, it is widely used in syngeneic and human tumor xenograft models to assess checkpoint inhibitor efficacy.
Q5: Are PD-1/PD-L1 humanized mice suitable for autoimmune disease studies?
A5: Yes, this model enables exploration of immune tolerance breakdown and PD-1/PD-L1 pathway dysregulation in autoimmunity.