
C57BL/6N-Krastm2Bcgen Trp53tm1Bcgen Pdx1tm1(icre)Bcgen/Bcgen • 113579

| Product name | B-KPC mice |
|---|---|
| Catalog number | 113579 |
| Strain name | C57BL/6N-Krastm2Bcgen Trp53tm1Bcgen Pdx1tm1(icre)Bcgen/Bcgen |
| Strain background | C57BL/6N |
| NCBI gene ID | 22059,27205,16653 (Mouse) |
| Aliases | bbl; bfy; bhy; p44; p53; Tp53; PC; Ly102; Pclp1; PCLP-1; Podxl1; ras; p21B; K-Ras; K-ras; Kras2; Ki-ras; Kras-2; K-Ras 2; c-K-ras; c-Ki-ras |
Gene targeting strategy for B-KPC mice.
A targeting vector was designed to place a G12D point mutation in exon 2 of the Kras gene, the loxP-flanked STOP element is located upstream of point mutations. The stop cassette prevents the expression of mutant Kras until it is removed by Cre mediated recombination of the Loxp sites, thus allowing expression of oncogenic Kras.
A targeting vector was designed to place a R172H point mutation in exon 5 of the Trp53 gene, the loxP-flanked STOP element is located upstream of point mutations. The stop cassette prevents the expression of mutant Trp53 until it is removed by Cre mediated recombination of the loxP sites, thus allowing expression of oncogenic Trp53.
The Pdx-driven expression cassette enables tissue-specific expression of Cre recombinase in the pancreas, excising the "Stop" sequence flanked by a pair of loxP sites, thereby initiating the expression of the Kras*G12D and Trp53*R172H genes.
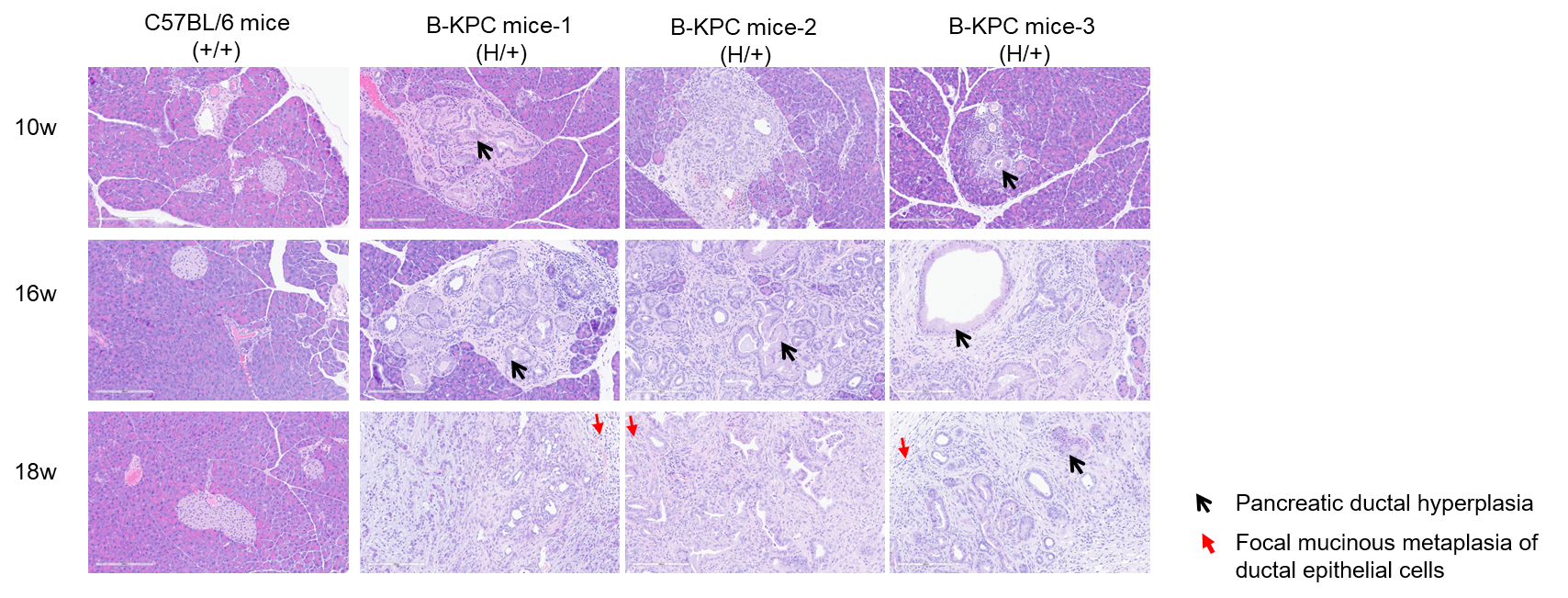
H&E staining of pancreas tissues in B-KPC mice. Pancreas tissues of C57BL/6 mice (+/+) (Female, 10w, 16w, 18w, n=3) and B-KPC mice (H/+) (Female, 10w, 16w, 18w, n=3) were collected and analyzed with H&E staining. The results show that B-KPC mice exhibited varying degrees of pancreatic ductal hyperplasia characterized by variable glandular lumen size, focal mucinous metaplasia of ductal epithelial cells, nuclear pleomorphism (unequal nuclear size), and the presence of pathological mitotic figures. Additionally, periductal fibrous stroma accompanied by inflammatory cell infiltration was observed. From 10 to 18 weeks of age, progressively worsened pathological alterations were observed, manifested as increased nuclear atypia, heightened tissue dysplasia, and expansion of tumor invasion. Based on these histopathological alterations, the findings are consistent with pancreatic ductal adenocarcinoma. The pancreatic tissues of wild-type mice at different weeks of age show no significant abnormalities.
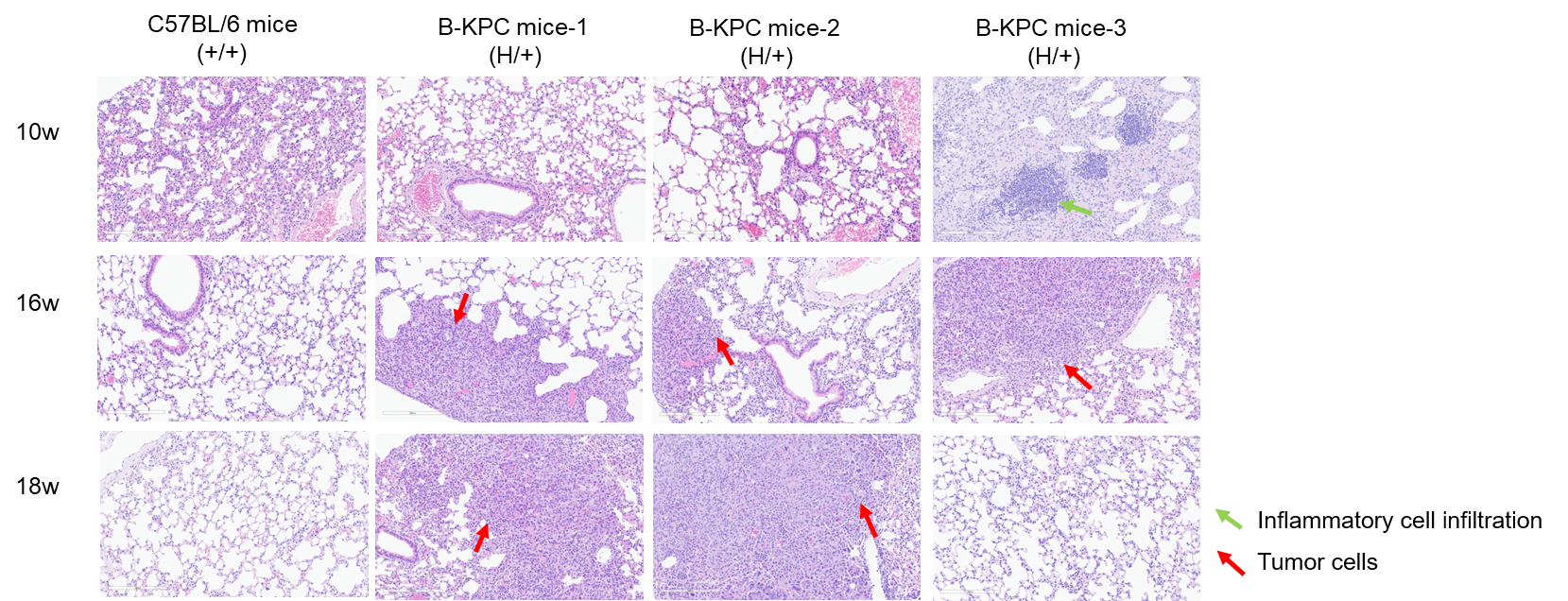
H&E staining of lung tissues in B-KPC mice. Lung tissues of C57BL/6 mice (+/+) (Female, 10w, 16w, 18w, n=3) and B-KPC mice (H/+) (Female, 10w, 16w, 18w, n=3) were collected and analyzed with H&E staining. At 10 weeks of age, only one animal exhibited thickened alveolar walls and inflammatory cell infiltration in the lungs, the lungs of all other mice showed no significant abnormalities. At 16 weeks of age, tumor cells were identified in the lungs of two animals, suggesting metastatic tumor, one animal exhibited inflammatory cell infiltration in the lungs. At 18 weeks of age, tumor cells were identified in the lungs of two animals, suggesting metastatic tumor. The lung tissues of wild-type mice at different weeks of age show no significant abnormalities.
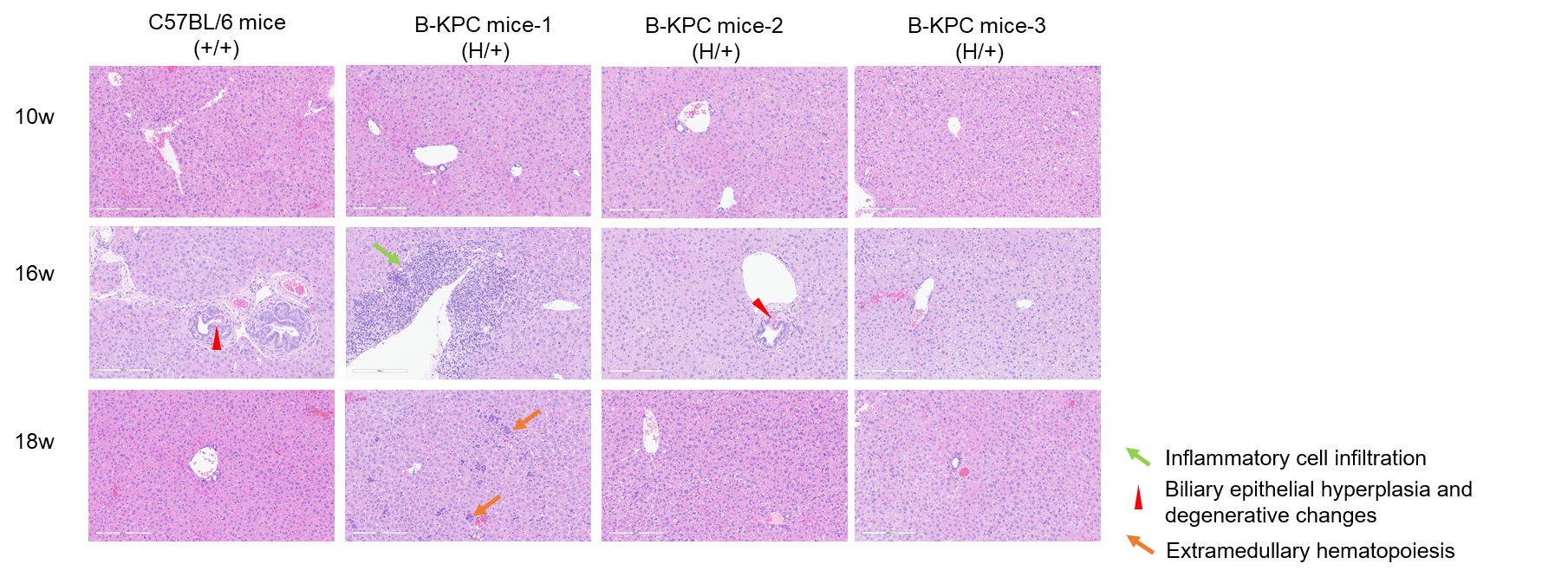
H&E staining of liver tissues in B-KPC mice. Liver tissues of C57BL/6 mice (+/+) (Female, 10w, 16w, 18w, n=3) and B-KPC mice (H/+) (Female, 10w, 16w, 18w, n=3) were collected and analyzed with H&E staining. At 10 weeks of age, liver tissues of B-KPC mice showed no significant abnormalities. At 16 weeks of age, biliary epithelial hyperplasia and degenerative changes were identified in the livers of two animals, while perivascular inflammatory cell infiltration was observed in the liver of one other animal. At 18 weeks of age, extramedullary hematopoiesis was noted in the liver of one, the livers of the remaining two animals exhibited no significant abnormalities. The liver tissues of wild-type mice at different weeks of age show no significant abnormalities.